Recent Progress in Synthetic Applications of Hypervalent Iodine(III) Reagents
- PMID: 39269928
- PMCID: PMC11468727
- DOI: 10.1021/acs.chemrev.4c00303
Recent Progress in Synthetic Applications of Hypervalent Iodine(III) Reagents
Abstract
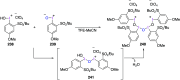
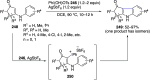
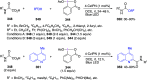
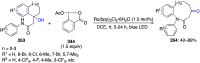
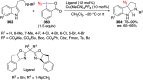
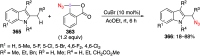
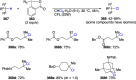
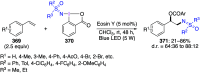
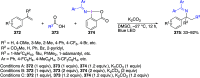
Hypervalent iodine(III) compounds have found wide application in modern organic chemistry as environmentally friendly reagents and catalysts. Hypervalent iodine reagents are commonly used in synthetically important halogenations, oxidations, aminations, heterocyclizations, and various oxidative functionalizations of organic substrates. Iodonium salts are important arylating reagents, while iodonium ylides and imides are excellent carbene and nitrene precursors. Various derivatives of benziodoxoles, such as azidobenziodoxoles, trifluoromethylbenziodoxoles, alkynylbenziodoxoles, and alkenylbenziodoxoles have found wide application as group transfer reagents in the presence of transition metal catalysts, under metal-free conditions, or using photocatalysts under photoirradiation conditions. Development of hypervalent iodine catalytic systems and discovery of highly enantioselective reactions using chiral hypervalent iodine compounds represent a particularly important recent achievement in the field of hypervalent iodine chemistry. Chemical transformations promoted by hypervalent iodine in many cases are unique and cannot be performed by using any other common, non-iodine-based reagent. This review covers literature published mainly in the last 7-8 years, between 2016 and 2024.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

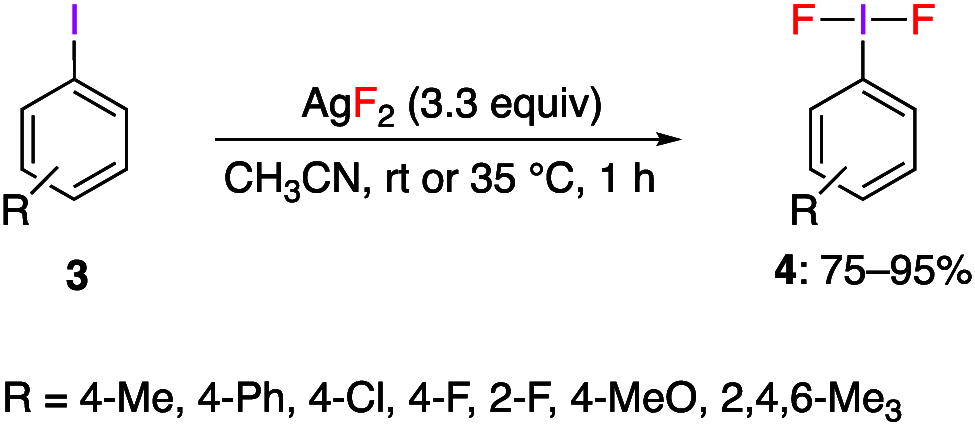
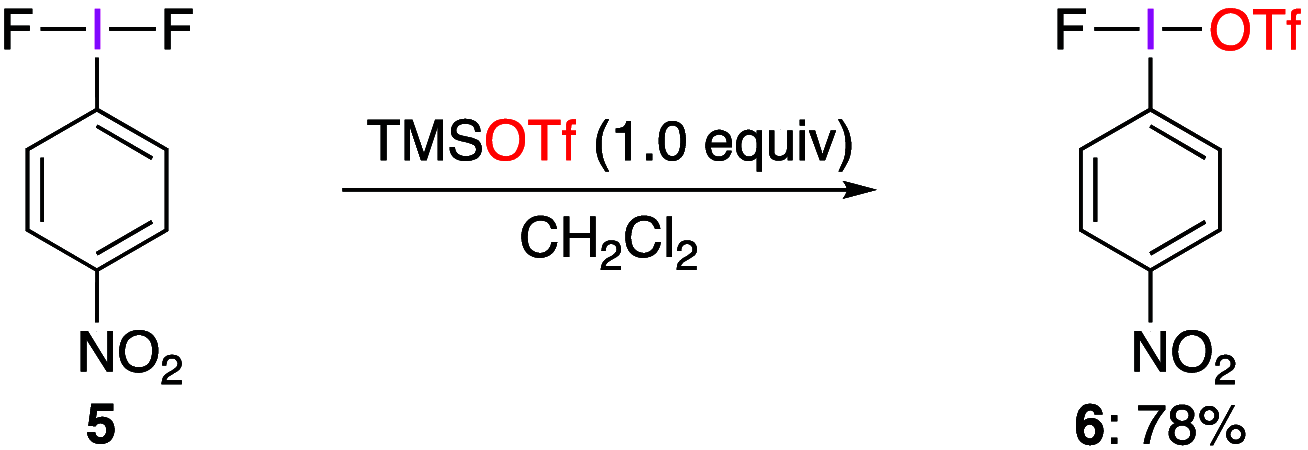


















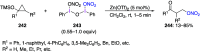
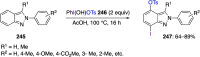







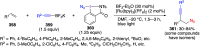






Similar articles
-
Iodine(III) Reagents in Radical Chemistry.Acc Chem Res. 2017 Jul 18;50(7):1712-1724. doi: 10.1021/acs.accounts.7b00148. Epub 2017 Jun 21. Acc Chem Res. 2017. PMID: 28636313 Free PMC article.
-
Advances in Synthetic Applications of Hypervalent Iodine Compounds.Chem Rev. 2016 Mar 9;116(5):3328-435. doi: 10.1021/acs.chemrev.5b00547. Epub 2016 Feb 10. Chem Rev. 2016. PMID: 26861673
-
Hypervalent iodine-catalyzed oxidative functionalizations including stereoselective reactions.Chem Asian J. 2014 Apr;9(4):950-71. doi: 10.1002/asia.201301582. Epub 2014 Feb 12. Chem Asian J. 2014. PMID: 24523252
-
Aryl-, Akynyl-, and Alkenylbenziodoxoles: Synthesis and Synthetic Applications.Molecules. 2023 Feb 24;28(5):2136. doi: 10.3390/molecules28052136. Molecules. 2023. PMID: 36903382 Free PMC article. Review.
-
Hypervalent Iodine Reagents in Palladium-Catalyzed Oxidative Cross-Coupling Reactions.Front Chem. 2020 Sep 29;8:705. doi: 10.3389/fchem.2020.00705. eCollection 2020. Front Chem. 2020. PMID: 33134246 Free PMC article. Review.
References
-
- Zhdankin V. V.Hypervalent Iodine Chemistry: Preparation, Structure and Synthetic Application of Polyvalent Iodine Compounds; John Wiley & Sons Ltd: Chichester (UK), 2014.
-
- Wirth T.Hypervalent Iodine Chemistry: Modern Developments in Organic Synthesis. Top. Curr. Chem. 373; Springer, 2016.
-
- Olofsson B.; Marek I.; Rappoport Z.. Patai’s Chemistry of Functional Groups: The Chemistry of Hypervalent Halogen Compounds; Wiley: Chichester, UK, 2019.
-
- Ishihara K.; Muñiz K.. Iodine Catalysis in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2022.
-
- Simonet-Davin R.; Waser J. Azidation with Hypervalent Iodine Reagents. Synthesis 2023, 55, 1652–1661. 10.1055/a-1966-4974. - DOI
Publication types
LinkOut - more resources
Full Text Sources

