Natural 7,8-secolignans from Schisandra sphenanthera fruit potently inhibit SARS-CoV-2 3CLpro and inflammation
- PMID: 39262656
- PMCID: PMC11384954
- DOI: 10.1016/j.jtcme.2024.01.005
Natural 7,8-secolignans from Schisandra sphenanthera fruit potently inhibit SARS-CoV-2 3CLpro and inflammation
Abstract
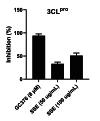
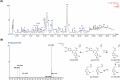
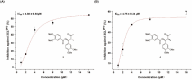
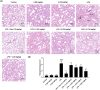
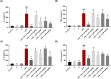
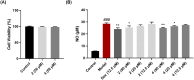
The coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), turned into a global pandemic, and there remains an urgent demand for specific/targeted drugs for the disease. The 3C-like protease (3CLpro) is a promising target for developing anti-coronavirus drugs. Schisandra sphenanthera fruit is a well-known traditional Chinese medicine (TCM) with good antiviral activity. This study found that the ethanolic extract displayed a significant inhibitory effect against SARS-CoV-2 3CLpro. Forty-four compounds were identified in this extract using ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF/MS). Combining molecular docking and in vitro experiments, we found that two epimeric 7,8-secolignans, rel-(1S,2R)-1-(3,4-dimethoxyphenyl)-2-methyl-3-oxobutyl-3,4-dimethoxybenzoate (2) and rel-(1S,2S)-1-(3,4-dimethoxyphenyl)-2-methyl-3-oxobutyl-3,4-dimethoxybenzoate (4), potently inhibited 3CLpro with IC50 values of 4.88 ± 0.60 μM and 4.75 ± 0.34 μM, respectively. Moreover, in vivo and in vitro experiments indicated that compounds 2 and 4 were potent in regulating the inflammatory response and preventing lung injury. Our findings indicate that compounds 2 and 4 may emerge as promising SARS-CoV-2 inhibitors via 3CLpro inhibition and anti-inflammatory mechanisms.
Keywords: 3C-like protease; 7,8-Secolignans; COVID-19; Inflammation; SARS-CoV-2; Schisandra sphenanthera fruit; UPLC-Q/TOF-MS.
© 2024 Center for Food and Biomolecules, National Taiwan University. Production and hosting by Elsevier Taiwan LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Effects of diarylbutane lignans from Schisandra chinensis fruit on SARS-CoV-2 3CLpro and PLpro and their in vitro anti-inflammatory properties.Phytomed Plus. 2023 May;3(2):100432. doi: 10.1016/j.phyplu.2023.100432. Epub 2023 Mar 11. Phytomed Plus. 2023. PMID: 36968623 Free PMC article.
-
Discovery and characterization of the covalent SARS-CoV-2 3CLpro inhibitors from Ginkgo biloba extract via integrating chemoproteomic and biochemical approaches.Phytomedicine. 2023 Jun;114:154796. doi: 10.1016/j.phymed.2023.154796. Epub 2023 Mar 29. Phytomedicine. 2023. PMID: 37037086 Free PMC article.
-
Identification of Darunavir Derivatives for Inhibition of SARS-CoV-2 3CLpro.Int J Mol Sci. 2022 Dec 16;23(24):16011. doi: 10.3390/ijms232416011. Int J Mol Sci. 2022. PMID: 36555652 Free PMC article.
-
The SARS-CoV-2 main protease (Mpro): Structure, function, and emerging therapies for COVID-19.MedComm (2020). 2022 Jul 14;3(3):e151. doi: 10.1002/mco2.151. eCollection 2022 Sep. MedComm (2020). 2022. PMID: 35845352 Free PMC article. Review.
-
Design and Evaluation of Anti-SARS-Coronavirus Agents Based on Molecular Interactions with the Viral Protease.Molecules. 2020 Aug 27;25(17):3920. doi: 10.3390/molecules25173920. Molecules. 2020. PMID: 32867349 Free PMC article. Review.
References
-
- WHO. World Health Organization WHO coronavirus disease (COVID-19) Dashboard [EB/OL] 2022. https://covid19.who.int
LinkOut - more resources
Full Text Sources
Miscellaneous
