Tumor-associated neutrophils upregulate Nectin2 expression, creating the immunosuppressive microenvironment in pancreatic ductal adenocarcinoma
- PMID: 39261943
- PMCID: PMC11389261
- DOI: 10.1186/s13046-024-03178-6
Tumor-associated neutrophils upregulate Nectin2 expression, creating the immunosuppressive microenvironment in pancreatic ductal adenocarcinoma
Abstract
Background: Tumor-associated neutrophils (TANs) constitute an abundant component among tumor-infiltrating immune cells and have recently emerged as a critical player in pancreatic ductal adenocarcinoma (PDAC) progression. This study aimed to elucidate the pro-tumor mechanisms of TAN and identify a novel target for effective immunotherapy against PDAC.
Methods: Microarray and cytokine array analyses were performed to identify the mechanisms underlying the function of TANs. Human and mouse TANs were obtained from differentiated HL-60 cells and orthotopically transplanted PDAC tumors, respectively. The interactions of TANs with cancer and cytotoxic T-cells were evaluated through in vitro co-culture and in vivo orthotopic or subcutaneous models. Single-cell transcriptomes from patients with PDAC were analyzed to validate the cellular findings.
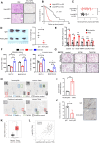
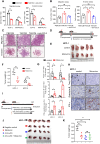
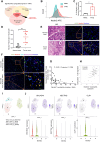
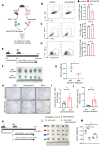
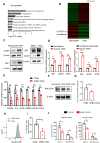
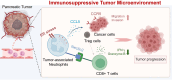
Results: Increased neutrophil infiltration in the tumor microenvironment was associated with poor survival in patients with PDAC. TANs secreted abundant amounts of chemokine ligand 5 (CCL5), subsequently enhancing cancer cell migration and invasion. TANs subpopulations negatively correlated with cytotoxic CD8+ T-cell infiltration in PDAC and promoted T-cell dysfunction. TANs upregulated the membranous expression of Nectin2, which contributed to CD8+ T-cell exhaustion. Blocking Nectin2 improved CD8+ T-cell function and suppressed tumor progression in the mouse model. Single-cell analysis of human PDAC revealed two immunosuppressive TANs phenotypes: Nectin2+ TANs and OLR1+ TANs. Endoplasmic reticulum stress regulated the protumor activities in TANs.
Conclusions: TANs enhance PDAC progression by secreting CCL5 and upregulating Nectin2. Targeting the immune checkpoint Nectin2 could represent a novel strategy to enhance immunotherapy efficacy in PDAC.
Keywords: Chemokine ligand 5; Nectin2; Pancreatic cancer; Tumor immune microenvironment; Tumor-associated neutrophils.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Single-cell RNA-seq analysis reveals BHLHE40-driven pro-tumour neutrophils with hyperactivated glycolysis in pancreatic tumour microenvironment.Gut. 2023 May;72(5):958-971. doi: 10.1136/gutjnl-2021-326070. Epub 2022 Jun 10. Gut. 2023. PMID: 35688610 Free PMC article.
-
Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer.J Hematol Oncol. 2019 Nov 27;12(1):124. doi: 10.1186/s13045-019-0822-6. J Hematol Oncol. 2019. PMID: 31771616 Free PMC article.
-
SIN3B Loss Heats up Cold Tumor Microenvironment to Boost Immunotherapy in Pancreatic Cancer.Adv Sci (Weinh). 2024 Nov;11(43):e2402244. doi: 10.1002/advs.202402244. Epub 2024 Sep 24. Adv Sci (Weinh). 2024. PMID: 39316363 Free PMC article.
-
Neutrophil in the Pancreatic Tumor Microenvironment.Biomolecules. 2021 Aug 7;11(8):1170. doi: 10.3390/biom11081170. Biomolecules. 2021. PMID: 34439836 Free PMC article. Review.
-
Neutrophils in pancreatic ductal adenocarcinoma: bridging preclinical insights to clinical prospects for improved therapeutic strategies.Expert Rev Clin Immunol. 2024 Aug;20(8):945-958. doi: 10.1080/1744666X.2024.2348605. Epub 2024 May 7. Expert Rev Clin Immunol. 2024. PMID: 38690749 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

