Infrared spectral profiling of demyelinating activity in multiple sclerosis brain tissue
- PMID: 39256864
- PMCID: PMC11385516
- DOI: 10.1186/s40478-024-01854-4
Infrared spectral profiling of demyelinating activity in multiple sclerosis brain tissue
Abstract
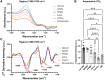
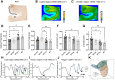
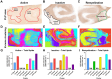
Multiple sclerosis (MS) is a leading cause of non-traumatic disability in young adults. The highly dynamic nature of MS lesions has made them difficult to study using traditional histopathology due to the specificity of current stains. This requires numerous stains to track and study demyelinating activity in MS. Thus, we utilized Fourier transform infrared (FTIR) spectroscopy to generate holistic biomolecular profiles of demyelinating activities in MS brain tissue. Multivariate analysis can differentiate MS tissue from controls. Analysis of the absorbance spectra shows profound reductions of lipids, proteins, and phosphate in white matter lesions. Changes in unsaturated lipids and lipid chain length indicate oxidative damage in MS brain tissue. Altered lipid and protein structures suggest changes in MS membrane structure and organization. Unique carbohydrate signatures are seen in MS tissue compared to controls, indicating altered metabolic activities. Cortical lesions had increased olefinic lipid content and abnormal membrane structure in normal appearing MS cortex compared to controls. Our results suggest that FTIR spectroscopy can further our understanding of lesion evolution and disease mechanisms in MS paving the way towards improved diagnosis, prognosis, and development of novel therapeutics.
Keywords: Biomolecular profiling; Cortex; Demyelinating activity; Fourier transform infrared spectroscopy; Human brain tissue; Lesions; Multiple sclerosis; Sparse partial least squares-discriminant analysis; White matter.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
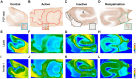
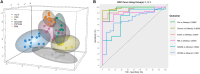
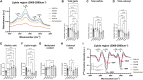
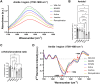
Similar articles
-
Biomolecular alterations detected in multiple sclerosis skin fibroblasts using Fourier transform infrared spectroscopy.Front Cell Neurosci. 2023 Sep 4;17:1223912. doi: 10.3389/fncel.2023.1223912. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37744877 Free PMC article.
-
Brain macrophages acquire distinct transcriptomes in multiple sclerosis lesions and normal appearing white matter.Acta Neuropathol Commun. 2022 Jan 28;10(1):8. doi: 10.1186/s40478-021-01306-3. Acta Neuropathol Commun. 2022. PMID: 35090578 Free PMC article.
-
White matter changes in paediatric multiple sclerosis and monophasic demyelinating disorders.Brain. 2017 May 1;140(5):1300-1315. doi: 10.1093/brain/awx041. Brain. 2017. PMID: 28334875
-
Chemical analysis of multiple sclerosis lesions by FT-IR microspectroscopy.Free Radic Biol Med. 1998 Jul 1;25(1):33-41. doi: 10.1016/s0891-5849(98)00019-7. Free Radic Biol Med. 1998. PMID: 9655519
-
Remodeling of the interstitial extracellular matrix in white matter multiple sclerosis lesions: Implications for remyelination (failure).J Neurosci Res. 2020 Jul;98(7):1370-1397. doi: 10.1002/jnr.24582. Epub 2020 Jan 21. J Neurosci Res. 2020. PMID: 31965607 Review.
References
-
- Ali MHM, Rakib F, Abdelalim EM, Limbeck A, Mall R, Ullah E, Mesaeli N, McNaughton D, Ahmed T, Al-Saad K (2018) Fourier-transform infrared imaging spectroscopy and laser ablation -ICPMS new vistas for biochemical analyses of ischemic stroke in rat brain. Front Neurosci. 10.3389/fnins.2018.00647 10.3389/fnins.2018.00647 - DOI - PMC - PubMed
-
- Ali MHM, Rakib F, Nischwitz V, Ullah E, Mall R, Shraim AM, Ahmad MI, Ghouri ZK, McNaughton D, Küppers S et al (2018) Application of FTIR and LA-ICPMS spectroscopies as a possible approach for biochemical analyses of different rat brain regions. Appl Sci 8:243610.3390/app8122436 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

