Non-viral approaches in CAR-NK cell engineering: connecting natural killer cell biology and gene delivery
- PMID: 39256765
- PMCID: PMC11384716
- DOI: 10.1186/s12951-024-02746-4
Non-viral approaches in CAR-NK cell engineering: connecting natural killer cell biology and gene delivery
Abstract
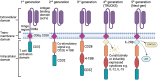
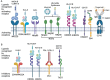
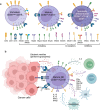
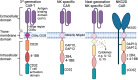
Natural Killer (NK) cells are exciting candidates for cancer immunotherapy with potent innate cytotoxicity and distinct advantages over T cells for Chimeric Antigen Receptor (CAR) therapy. Concerns regarding the safety, cost, and scalability of viral vectors has ignited research into non-viral alternatives for gene delivery. This review comprehensively analyses recent advancements and challenges with non-viral genetic modification of NK cells for allogeneic CAR-NK therapies. Non-viral alternatives including electroporation and multifunctional nanoparticles are interrogated with respect to CAR expression and translational responses. Crucially, the link between NK cell biology and design of drug delivery technologies are made, which is essential for development of future non-viral approaches. This review provides valuable insights into the current state of non-viral CAR-NK cell engineering, aimed at realising the full potential of NK cell-based immunotherapies.
Keywords: CAR-NK engineering; Cancer immunotherapy; Cell therapies; Drug delivery technologies; NK-specific CAR design; Nanoparticles; Natural killer cells; Non-viral gene delivery.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Chimeric antigen receptor (CAR) natural killer (NK)-cell therapy: leveraging the power of innate immunity.Br J Haematol. 2021 Apr;193(2):216-230. doi: 10.1111/bjh.17186. Epub 2020 Nov 20. Br J Haematol. 2021. PMID: 33216984 Free PMC article. Review.
-
Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy.J Hematol Oncol. 2020 Dec 7;13(1):168. doi: 10.1186/s13045-020-00998-9. J Hematol Oncol. 2020. PMID: 33287875 Free PMC article. Review.
-
Current Perspectives on "Off-The-Shelf" Allogeneic NK and CAR-NK Cell Therapies.Front Immunol. 2021 Dec 1;12:732135. doi: 10.3389/fimmu.2021.732135. eCollection 2021. Front Immunol. 2021. PMID: 34925314 Free PMC article. Review.
-
Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy.J Hematol Oncol. 2021 May 1;14(1):73. doi: 10.1186/s13045-021-01083-5. J Hematol Oncol. 2021. PMID: 33933160 Free PMC article. Review.
-
CAR-NK Cells: From Natural Basis to Design for Kill.Front Immunol. 2021 Dec 14;12:707542. doi: 10.3389/fimmu.2021.707542. eCollection 2021. Front Immunol. 2021. PMID: 34970253 Free PMC article. Review.
References
-
- Karakostas P, Panoskaltsis N, Mantalaris A, Georgiadis MC. Optimization of CAR T-cell therapies supply chains. Comput Chem Eng. 2020;139: 106913. 10.1016/j.compchemeng.2020.106913.10.1016/j.compchemeng.2020.106913 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

