Letrozole co-treatment in an antagonist protocol for overweight women undergoing IVF treatment: a retrospective study
- PMID: 39256667
- PMCID: PMC11386352
- DOI: 10.1186/s12884-024-06795-3
Letrozole co-treatment in an antagonist protocol for overweight women undergoing IVF treatment: a retrospective study
Abstract
Background: Overweight women undergoing IVF treatment have lower success rates. Letrozole, an aromatase inhibitor, has been used as an adjunct for IVF treatment, but its specific effects in overweight women have not been investigated. This study was to explore the effects of letrozole co-treatment in an antagonist protocol for overweight infertile women undergoing IVF treatment.
Methods: This retrospective cohort study included overweight infertile women who underwent IVF/ICSI treatment and fresh embryo transfer (ET), with or without letrozole co-treatment in an antagonist protocol, from 2007 to 2021 at Shanghai Ninth People's Hospital (Shanghai, China). A total of 704 overweight infertile women were included: 585 women were in the antagonist group, and 119 women were in the letrozole co-treatment group. The primary outcome was the live birth rate after fresh ET. Propensity score-based patient-matching was employed to balance the covariates between the groups. Multivariate logistic regression analysis was also performed to estimate odds ratio (OR) and 95% confidence interval (CI) for association of letrozole co-treatment and the live birth outcome.
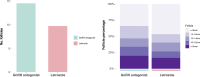
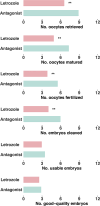
Results: Letrozole co-treatment induced significant changes in hormonal profile on the trigger day. The letrozole group exhibited a decrease in the total number of follicles compared to the antagonist group, but a higher proportion of large follicles at oocyte retrieval (P < 0.05). The quantity and quality of embryos were comparable between the two groups (P > 0.05). The letrozole co-treatment group had a significantly higher live birth rate than the control group (38.7% vs. 22.6%, P = 0.026). With multivariate logistic regression analysis, letrozole co-treatment was associated with higher odds of live birth after adjusting for potential confounding factors (adjusted OR = 2.00, 95% CI = 1.17-3.39, P = 0.011). Letrozole presented no significant associations with obstetrical or neonatal complications (P > 0.05).
Conclusion: Letrozole co-treatment in an antagonist protocol may offer potential benefits for overweight infertile women undergoing IVF treatment. Further research is warranted to validate these findings and explore the broader implications for letrozole co-treatment.
Keywords: Antagonist protocol; Embryo transfer; In vitro fertilization; Letrozole; Live birth; Obesity; Overweight.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Impact of letrozole co-treatment in an antagonist protocol for IVF/ICSI: a retrospective study.Reprod Biol Endocrinol. 2024 Oct 16;22(1):126. doi: 10.1186/s12958-024-01297-5. Reprod Biol Endocrinol. 2024. PMID: 39415184 Free PMC article.
-
The Efficacy of Letrozole Co-Treatment in an Antagonist Protocol for Women with Polycystic Ovary Syndrome Undergoing IVF: A Retrospective Study.Drug Des Devel Ther. 2024 Jul 8;18:2823-2835. doi: 10.2147/DDDT.S458608. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39006189 Free PMC article.
-
Impact of letrozole co-treatment during ovarian stimulation on oocyte yield, embryo development, and live birth rate in women with normal ovarian reserve: secondary outcomes from the RIOT trial.Hum Reprod. 2023 Nov 2;38(11):2154-2165. doi: 10.1093/humrep/dead182. Hum Reprod. 2023. PMID: 37699851 Clinical Trial.
-
Oral medications including clomiphene citrate or aromatase inhibitors with gonadotropins for controlled ovarian stimulation in women undergoing in vitro fertilisation.Cochrane Database Syst Rev. 2017 Nov 2;11(11):CD008528. doi: 10.1002/14651858.CD008528.pub3. Cochrane Database Syst Rev. 2017. PMID: 29096046 Free PMC article. Review.
-
Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist-assisted reproductive technology.Cochrane Database Syst Rev. 2014 Oct 31;2014(10):CD008046. doi: 10.1002/14651858.CD008046.pub4. Cochrane Database Syst Rev. 2014. PMID: 25358904 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

