Navitoclax safety, tolerability, and effect on biomarkers of senescence and neurodegeneration in aged nonhuman primates
- PMID: 39253182
- PMCID: PMC11382177
- DOI: 10.1016/j.heliyon.2024.e36483
Navitoclax safety, tolerability, and effect on biomarkers of senescence and neurodegeneration in aged nonhuman primates
Abstract
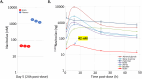
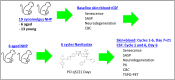
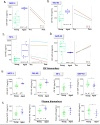
Alzheimer's disease (AD) is the most common global dementia and is universally fatal. Most late-stage AD disease-modifying therapies are intravenous and target amyloid beta (Aβ), with only modest effects on disease progression: there remains a high unmet need for convenient, safe, and effective therapeutics. Senescent cells (SC) and the senescence-associated secretory phenotype (SASP) drive AD pathology and increase with AD severity. Preclinical senolytic studies have shown improvements in neuroinflammation, tau, Aβ, and CNS damage; most were conducted in transgenic rodent models with uncertain human translational relevance. In this study, aged cynomolgus monkeys had significant elevation of biomarkers of senescence, SASP, and neurological damage. Intermittent treatment with the senolytic navitoclax induced modest reversible thrombocytopenia; no serious drug-related toxicity was noted. Navitoclax reduced several senescence and SASP biomarkers, with CSF concentrations sufficient for senolysis. Finally, navitoclax reduced TSPO-PET frontal cortex binding and showed trends of improvement in CSF biomarkers of neuroinflammation, neuronal damage, and synaptic dysfunction. Overall, navitoclax administration was safe and well tolerated in aged monkeys, inducing trends of biomarker changes relevant to human neurodegenerative disease.
© 2024 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Edward Greenberg, MD reports financial support was provided by AbbVie Inc. Edward Greenberg MD reports a relationship with AbbVie Inc that includes: employment, equity or stocks, and funding grants. EFG, MV, AS, DRR, YZ, JQW, DWW, EA, MH, CH, RD, MT, KO, WA, LD, HK, JSS, YH, KM, VZ, SY, JM, YF, AV, SW, MD, SJF, and HF are employees of AbbVie and may hold stock. KD was an employee of AbbVie at the time of the study and may hold stock. The design, study conduct, and all funding for this research was provided by AbbVie, the maker of navitoclax (ABT-263). AbbVie participated in the interpretation of data, review, and approval of the publication. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


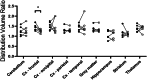
Similar articles
-
PTI-125 Reduces Biomarkers of Alzheimer's Disease in Patients.J Prev Alzheimers Dis. 2020;7(4):256-264. doi: 10.14283/jpad.2020.6. J Prev Alzheimers Dis. 2020. PMID: 32920628 Clinical Trial.
-
A navitoclax-loaded nanodevice targeting matrix metalloproteinase-3 for the selective elimination of senescent cells.Acta Biomater. 2024 Mar 1;176:405-416. doi: 10.1016/j.actbio.2024.01.002. Epub 2024 Jan 5. Acta Biomater. 2024. PMID: 38185231
-
The Senolytic Drug Navitoclax (ABT-263) Causes Trabecular Bone Loss and Impaired Osteoprogenitor Function in Aged Mice.Front Cell Dev Biol. 2020 May 20;8:354. doi: 10.3389/fcell.2020.00354. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32509782 Free PMC article.
-
Translating the Biology of Aging into New Therapeutics for Alzheimer's Disease: Senolytics.J Prev Alzheimers Dis. 2023;10(4):633-646. doi: 10.14283/jpad.2023.104. J Prev Alzheimers Dis. 2023. PMID: 37874084 Free PMC article. Review.
-
Senescence as an Amyloid Cascade: The Amyloid Senescence Hypothesis.Front Cell Neurosci. 2020 May 19;14:129. doi: 10.3389/fncel.2020.00129. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32508595 Free PMC article. Review.
References
-
- Jack Cox C.T. Biogen news release; 2024. Biogen to Realign Resources for Alzheimer's Disease Franchise.
-
- van Dyck C.H., Swanson C.J., Aisen P., Bateman R.J., Chen C., Gee M., Kanekiyo M., Li D., Reyderman L., Cohen S., Froelich L., Katayama S., Sabbagh M., Vellas B., Watson D., Dhadda S., Irizarry M., Kramer L.D., Iwatsubo T. Lecanemab in early Alzheimer's disease. N. Engl. J. Med. 2023;388:9–21. - PubMed
-
- Eisai Co L. Topline Results of Clarity AD: Conference for Media and Investors. 2022.
LinkOut - more resources
Full Text Sources

