Immunologic mediators profile in COVID-19 convalescence
- PMID: 39251702
- PMCID: PMC11384766
- DOI: 10.1038/s41598-024-71419-x
Immunologic mediators profile in COVID-19 convalescence
Abstract
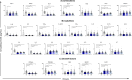
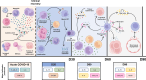
SARS-CoV-2 caused the pandemic situation experienced since the beginning of 2020, and many countries faced the rapid spread and severe form of the disease. Mechanisms of interaction between the virus and the host were observed during acute phase, but few data are available when related to immunity dynamics in convalescents. We conducted a longitudinal study, with 51 healthy donors and 62 COVID-19 convalescent patients, which these had a 2-month follow-up after symptoms recovery. Venous blood sample was obtained from all participants to measure blood count, subpopulations of monocytes, lymphocytes, natural killer cells and dendritic cells. Serum was used to measure cytokines, chemokines, growth factors, anti-N IgG and anti-S IgG/IgM antibodies. Statistic was performed by Kruskal-Wallis test, and linear regression with days post symptoms and antibody titers. All analysis had confidence interval of 95%. Less than 35% of convalescents were anti-S IgM+, while more than 80% were IgG+ in D30. Anti-N IgG decreased along time, with loss of seroreactivity of 13%. Eosinophil count played a distinct role on both antibodies during all study, and the convalescence was orchestrated by higher neutrophil-to-lymphocyte ratio and IL-15, but initial stages were marked by increase in myeloid DCs, B1 lymphocytes, inflammatory and patrolling monocytes, G-CSF and IL-2. Later convalescence seemed to change to cytotoxicity mediated by T lymphocytes, plasmacytoid DCs, VEGF, IL-9 and CXCL10. Anti-S IgG antibodies showed the longest perseverance and may be a better option for diagnosis. The inflammatory pattern is yet present on initial stage of convalescence, but quickly shifts to a reparative dynamic. Meanwhile eosinophils seem to play a role on anti-N levels in convalescence, although may not be the major causative agent. We must highlight the importance of immunological markers on acute clinical outcomes, but their comprehension to potentialize adaptive system must be explored to improve immunizations and further preventive policies.
Keywords: Antibody; Brazil; Immune hallmarks; Severe acute respiratory syndrome (SARS).
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Screening for SARS-CoV-2 antibodies in convalescent plasma in Brazil: Preliminary lessons from a voluntary convalescent donor program.Transfusion. 2020 Dec;60(12):2938-2951. doi: 10.1111/trf.16065. Epub 2020 Sep 16. Transfusion. 2020. PMID: 32935877 Free PMC article.
-
Kinetics of SARS-CoV-2 Specific and Neutralizing Antibodies over Seven Months after Symptom Onset in COVID-19 Patients.Microbiol Spectr. 2021 Oct 31;9(2):e0059021. doi: 10.1128/Spectrum.00590-21. Epub 2021 Sep 22. Microbiol Spectr. 2021. PMID: 34550000 Free PMC article.
-
Detection of Serum Cross-Reactive Antibodies and Memory Response to SARS-CoV-2 in Prepandemic and Post-COVID-19 Convalescent Samples.J Infect Dis. 2021 Oct 28;224(8):1305-1315. doi: 10.1093/infdis/jiab333. J Infect Dis. 2021. PMID: 34161567 Free PMC article.
-
SARS-CoV-2 antibodies: IgA correlates with severity of disease in early COVID-19 infection.J Med Virol. 2021 Sep;93(9):5409-5415. doi: 10.1002/jmv.27058. Epub 2021 May 12. J Med Virol. 2021. PMID: 33932299 Free PMC article.
-
Seroprevalence of SARS-CoV-2-specific antibodies in cancer outpatients in Madrid (Spain): A single center, prospective, cohort study and a review of available data.Cancer Treat Rev. 2020 Nov;90:102102. doi: 10.1016/j.ctrv.2020.102102. Epub 2020 Sep 1. Cancer Treat Rev. 2020. PMID: 32947121 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- PCTI-EMERGESAÚDE/AM-Chamada II Program #006/2020/Fundação de Amparo à Pesquisa do Estado do Amazonas
- POSGRAD Program #002/2023/Fundação de Amparo à Pesquisa do Estado do Amazonas
- POSGRAD Program #002/2024/Fundação de Amparo à Pesquisa do Estado do Amazonas
- Pró-Estado Program #002/2008/Fundação de Amparo à Pesquisa do Estado do Amazonas
- Pró-Estado Program #007/2018/Fundação de Amparo à Pesquisa do Estado do Amazonas
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

