Eligibility Criteria for Different Platinum-Based Chemotherapy Regimens in Metastatic Urothelial Carcinoma
- PMID: 39246966
- PMCID: PMC11380918
- DOI: 10.7759/cureus.66520
Eligibility Criteria for Different Platinum-Based Chemotherapy Regimens in Metastatic Urothelial Carcinoma
Abstract
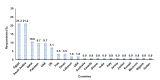
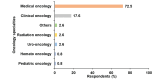
Introduction Bladder cancer is one of the most prevalent cancers worldwide, with significant morbidity and mortality rates. Treatment options for metastatic urothelial carcinoma (mUC) primarily include platinum-based chemotherapy. Cisplatin-based chemotherapy is conventionally used for treating mUC, but many patients are ineligible due to various factors such as poor performance status, creatinine clearance, neuropathy, and cardiac function. Carboplatin-based therapy is another alternative, which typically yields less favorable outcomes. Some centers use split-dose cisplatin for treating patients with comorbidities and impaired renal function, broadening cisplatin's spectrum. While eligibility criteria for full-dose cisplatin are well-established, those for split-dose cisplatin and carboplatin lack strong evidence. This study aims to assess the recommended criteria for full-dose cisplatin, split-dose cisplatin, and carboplatin regimens in real-world settings, including hematological parameters for patients with mUC. Methods A cross-sectional web-based survey was conducted among 136 oncologists from 21 countries, assessing criteria such as creatinine clearance, Eastern Cooperative Oncology Group (ECOG) performance status (PS), neurotoxicity, hearing loss, heart failure classification, and hematological parameters. Results The survey revealed diverse preferences among 113 oncologists treating mUC, regarding the eligibility criteria for each chemotherapy regimen with 81% prioritizing full-dose cisplatin, 21% split-dose cisplatin, and 14% carboplatin regimens. Criteria for all three regimens included specific thresholds. For full-dose cisplatin, the preferred criteria included creatinine clearance ≥60 mL/min, ECOG PS ≤1, grade 1 neuropathy, grade 1 deafness, New York Heart Association (NYHA) heart failure ≤class II with ≥50% cardiac ejection fraction, and normal blood parameters. Split-dose cisplatin criteria were creatinine clearance ≥40 mL/min, ECOG PS ≤2, grade 1 neuropathy, grade 1 deafness, NYHA heart failure ≤class II with ≥50% cardiac ejection fraction, and normal blood parameters. Carboplatin eligibility criteria were creatinine clearance ≥30, ECOG PS ≤2, grade ≤2 neuropathy, grade ≤2 deafness, NYHA heart failure ≤class II with ≥50% cardiac ejection fraction, and normal blood parameters. Hematological parameters were deemed crucial for all regimens, particularly stringent for carboplatin-based chemotherapy. Conclusion The study underscores the importance of renal function and hematological parameters in determining chemotherapy eligibility for patients with mUC. It highlights the importance of precise treatment criteria in mUC management, with hematological factors playing a significant role. Standardized criteria and further research are warranted to optimize treatment outcomes and minimize adverse events associated with chemotherapy regimens. Understanding the preferences of oncologists globally can facilitate tailored treatment approaches and improve patient care in the management of mUC.
Keywords: carboplatin-based therapy; cisplatin-based chemotherapy; eligibility criteria; hematological parameters; metastatic urothelial carcinoma.
Copyright © 2024, Azam et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: This study was funded by Pfizer/Merck Alliance, with an additional medical writing services grant. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
Similar articles
-
Real-World Analysis of Treatment Patterns and Platinum-Based Treatment Eligibility of Patients With Metastatic Urothelial Cancer in 5 European Countries.Clin Genitourin Cancer. 2024 Feb;22(1):e136-e147.e1. doi: 10.1016/j.clgc.2023.09.010. Epub 2023 Oct 2. Clin Genitourin Cancer. 2024. PMID: 37945404
-
Split-Dose Cisplatin in Patients With Locally Advanced or Metastatic Urothelial Carcinoma: A Systematic Literature Review and Network Meta-Analysis.Clin Genitourin Cancer. 2024 Jul 25;22(6):102176. doi: 10.1016/j.clgc.2024.102176. Online ahead of print. Clin Genitourin Cancer. 2024. PMID: 39260094
-
Comparative Study of the Safety and Efficacy of First-Line Cisplatin and Carboplatin Chemotherapy in Elderly Patients with Metastatic Urothelial Carcinoma.Oncology. 2020;98(3):146-153. doi: 10.1159/000504393. Epub 2019 Dec 3. Oncology. 2020. PMID: 31794969
-
Treatment of patients with metastatic urothelial cancer "unfit" for Cisplatin-based chemotherapy.J Clin Oncol. 2011 Jun 10;29(17):2432-8. doi: 10.1200/JCO.2011.34.8433. Epub 2011 May 9. J Clin Oncol. 2011. PMID: 21555688 Review.
-
Adverse events of different chemotherapy regimens in the first-line treatment of patients with advanced or metastatic urothelial cancer: A systematic review and network meta-analysis of randomized controlled trials.Semin Oncol. 2021 Jun;48(3):181-192. doi: 10.1053/j.seminoncol.2021.09.005. Epub 2021 Oct 2. Semin Oncol. 2021. PMID: 34749886 Review.
References
-
- World Cancer Research Fund International - Bladder Cancer Statistics. [ Feb; 2024 ]. 2020. https://www.wcrf.org/cancer-trends/bladder-cancer-statistics/ https://www.wcrf.org/cancer-trends/bladder-cancer-statistics/
-
- Non-urothelial and urothelial variants of bladder cancer. Yu EM, Belay S, Li W, Aragon-Ching JB. Cancer Treat Res Commun. 2022;33:100661. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources