Repurposed Drugs during the Outbreak of Pandemic COVID-19: A Mini-Review on Their Molecular Structures and Hit-and-Trial Results
- PMID: 39246499
- PMCID: PMC11375728
- DOI: 10.1021/acsomega.4c05357
Repurposed Drugs during the Outbreak of Pandemic COVID-19: A Mini-Review on Their Molecular Structures and Hit-and-Trial Results
Abstract
One of the most significant threats to global public health in the 21st century is the novel coronavirus disease (COVID-19) caused by SARS-CoV-2. It rapidly turned into a global pandemic after it was identified in late 2019, and the World Health Organization announced the end of the pandemic on May 5, 2023. Current strategies for managing this disease include vaccination and repurposing antimalarial and antibiotic medications to alleviate symptoms like fever and throat pain, which are associated with acute respiratory distress syndrome (ARDS). Antiviral drugs such as chloroquine, hydroxychloroquine, azithromycin, remdesivir, and favipiravir have been repurposed for the treatment of COVID-19. They were previously recommended for treating SARS-CoV and MERS-CoV. However, the inefficacy and adverse side effects of these repurposed drugs led to a decrease in their widespread use in treating COVID-19 patients. The lack of approved drugs for combating this coronavirus and its unpredictable variants remains a significant challenge.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
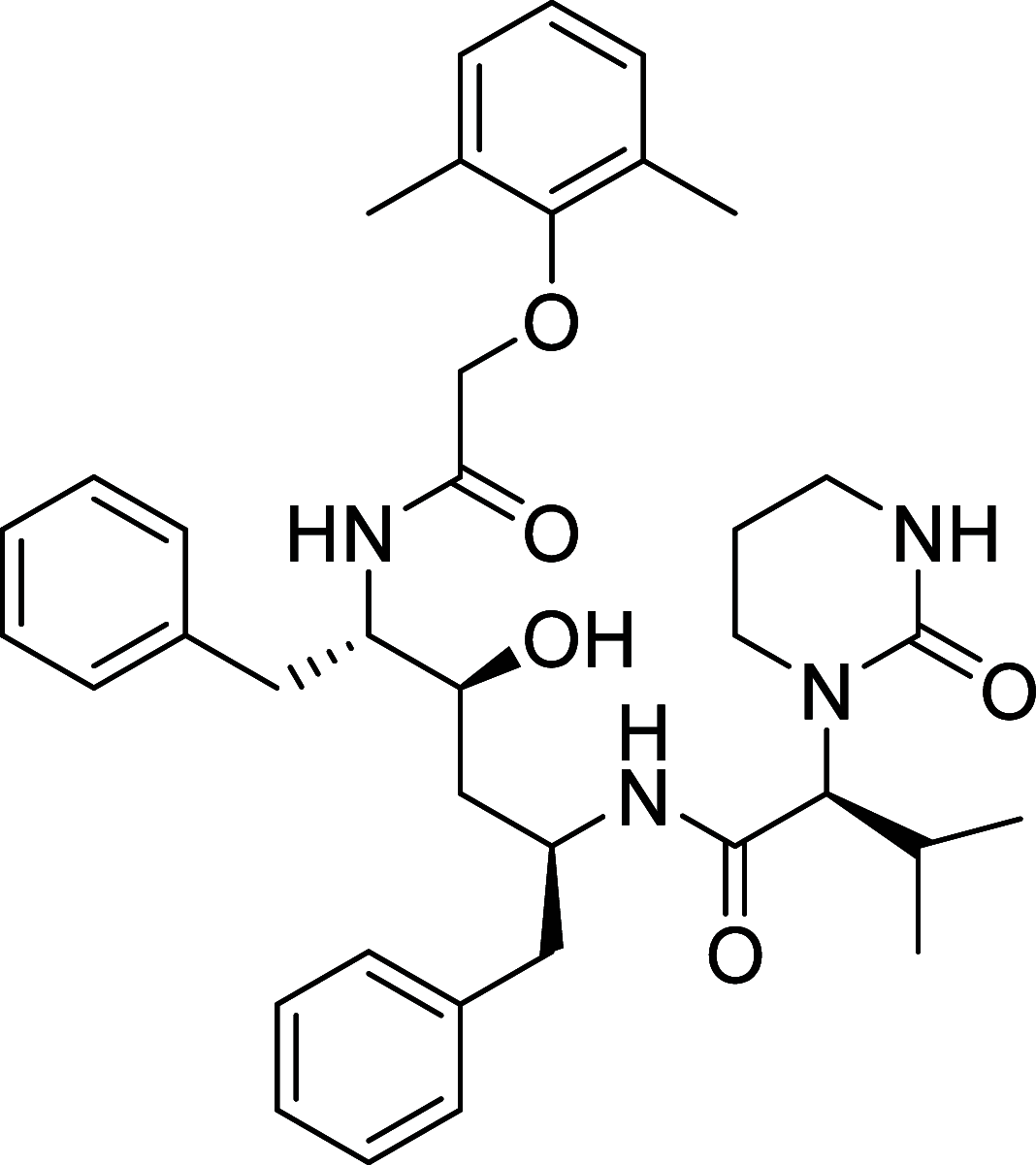
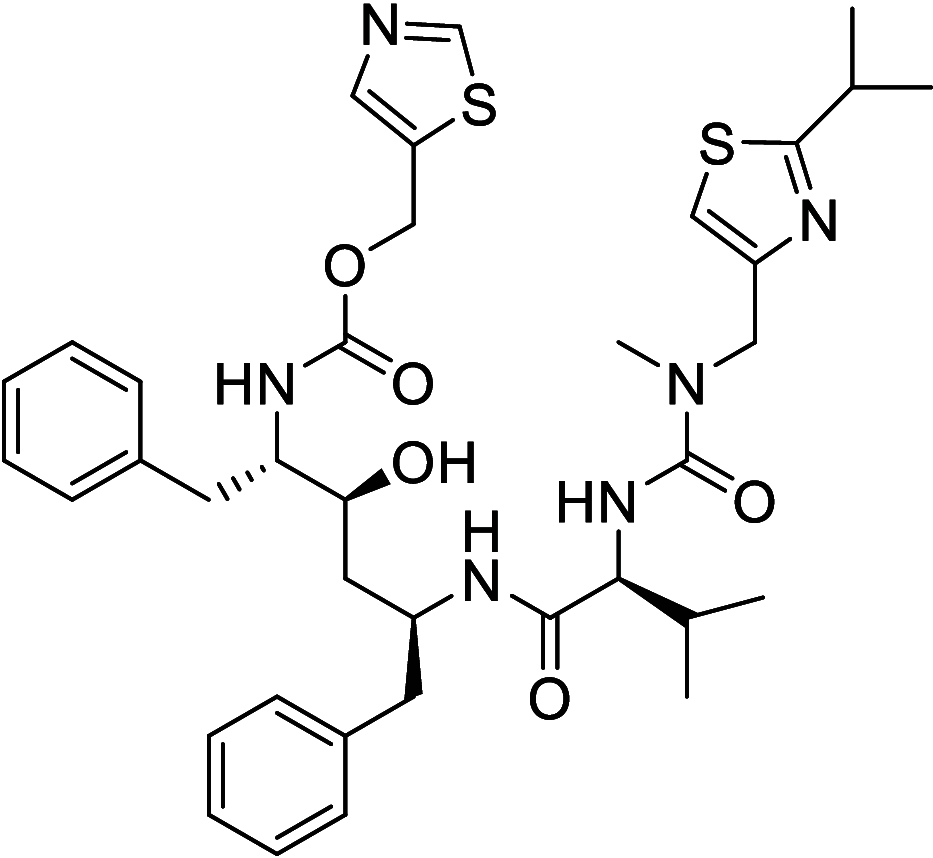
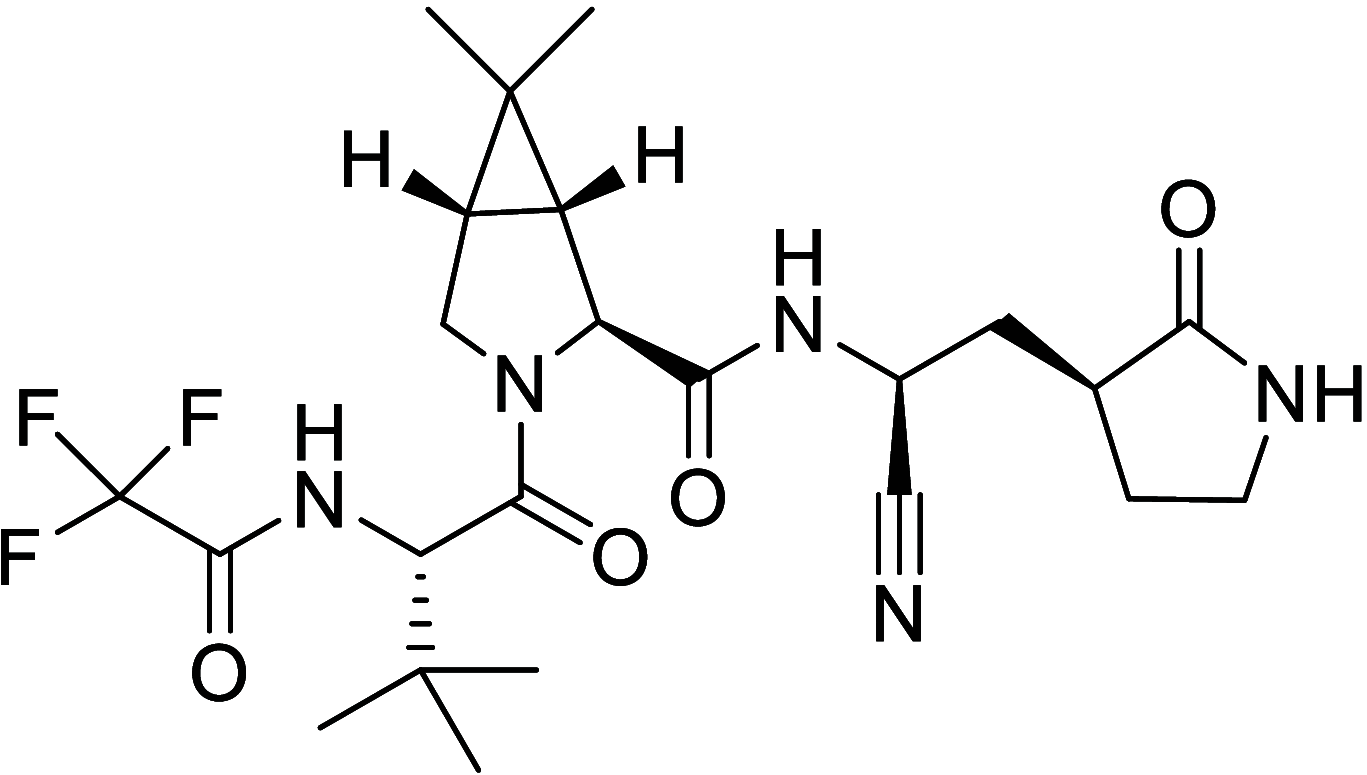
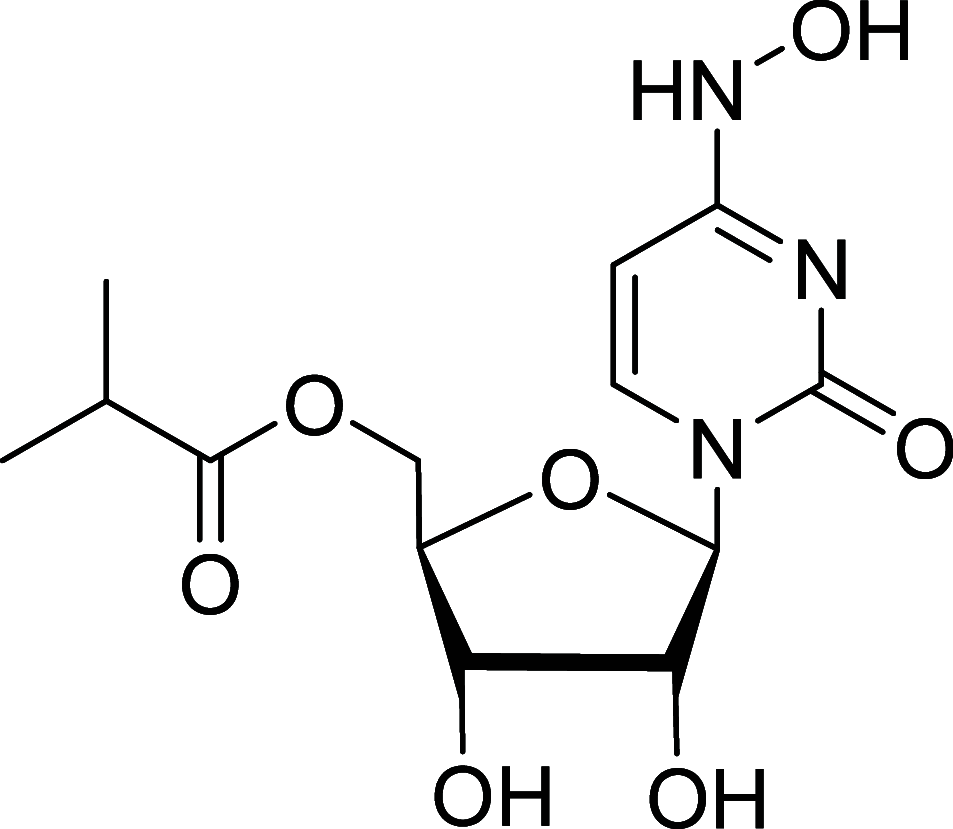
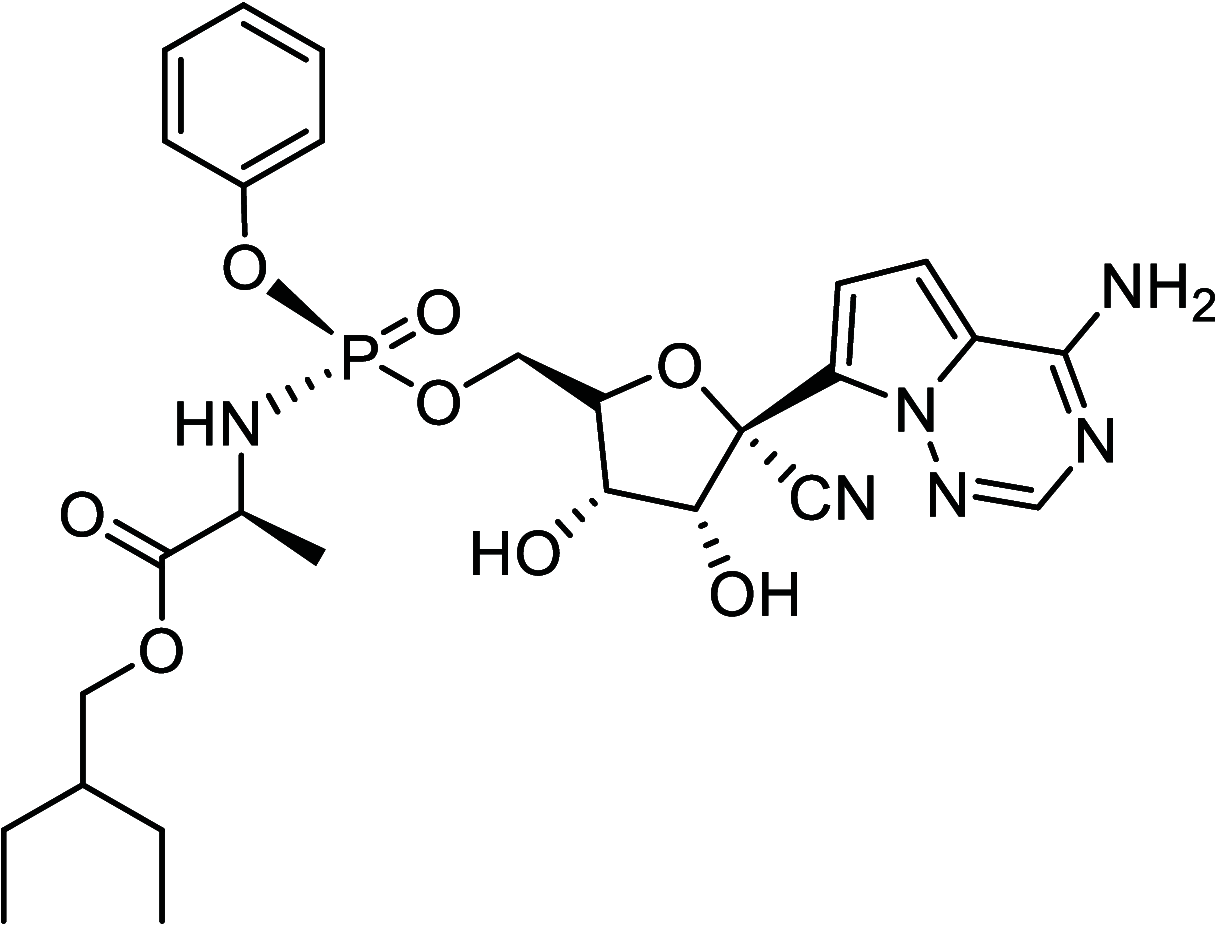
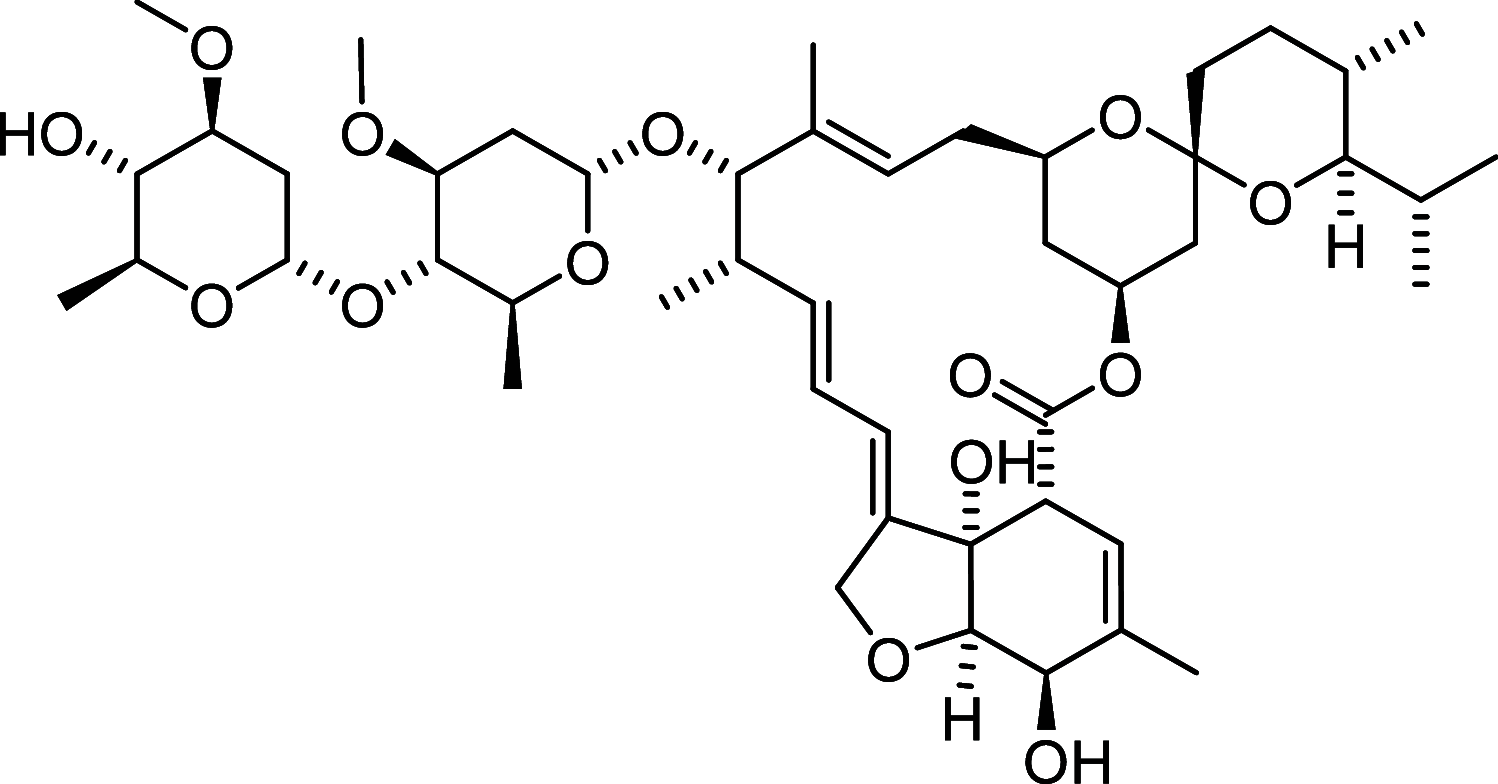
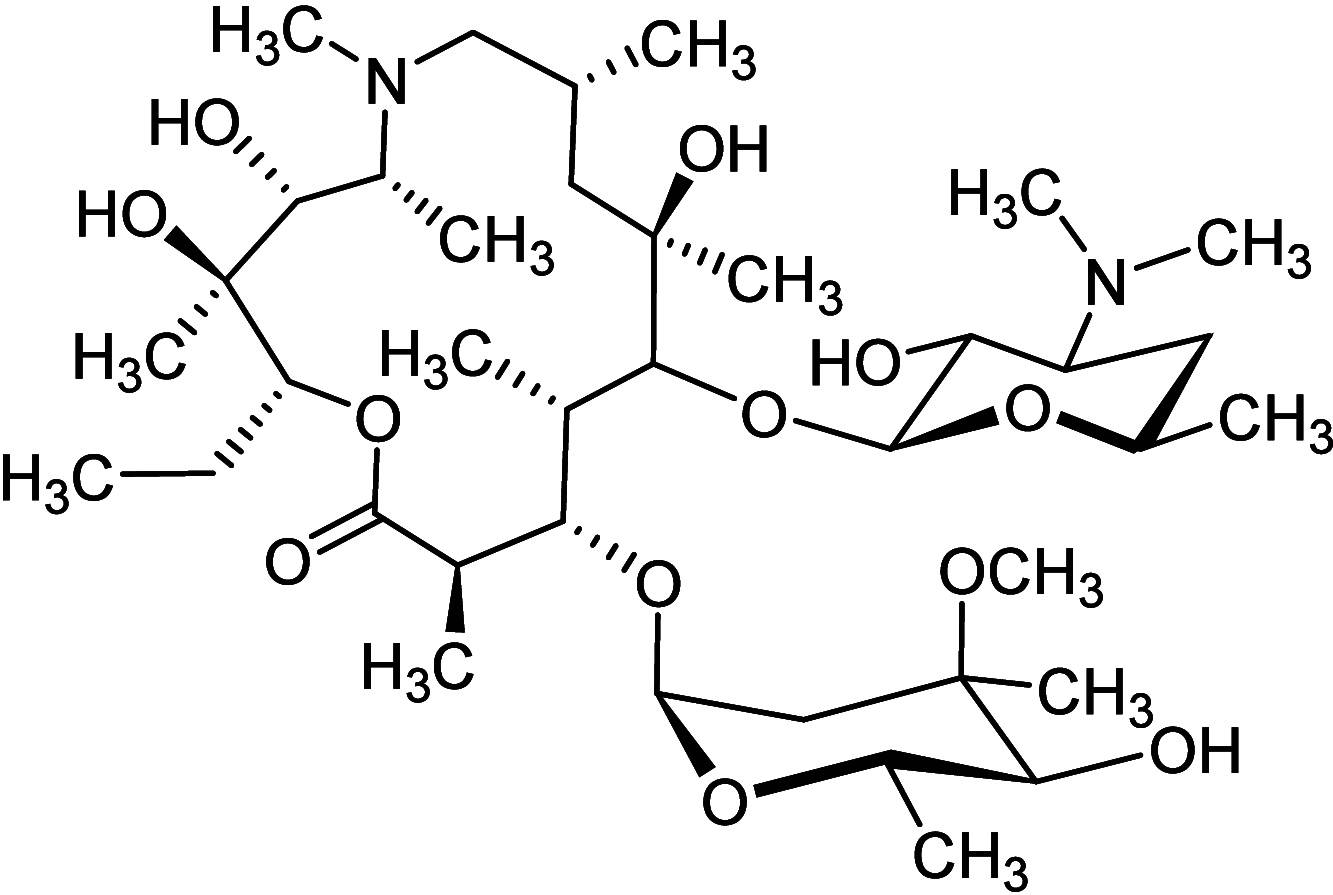
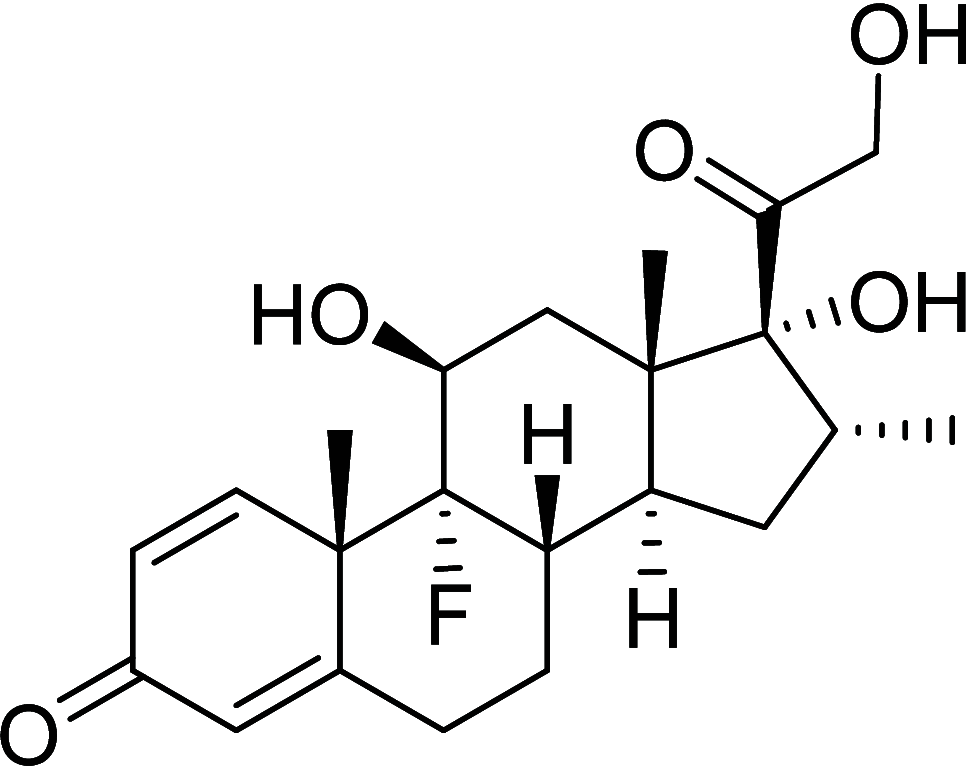
Similar articles
-
Current Strategies of Antiviral Drug Discovery for COVID-19.Front Mol Biosci. 2021 May 13;8:671263. doi: 10.3389/fmolb.2021.671263. eCollection 2021. Front Mol Biosci. 2021. PMID: 34055887 Free PMC article. Review.
-
Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2.Biomed Pharmacother. 2020 Nov;131:110668. doi: 10.1016/j.biopha.2020.110668. Epub 2020 Aug 24. Biomed Pharmacother. 2020. PMID: 32861965 Free PMC article. Review.
-
Impact of repurposed drugs on the symptomatic COVID-19 patients.J Infect Public Health. 2021 Jan;14(1):24-38. doi: 10.1016/j.jiph.2020.11.009. Epub 2020 Dec 7. J Infect Public Health. 2021. PMID: 33341481 Free PMC article. Review.
-
The journey of antimalarial drugs against SARS-CoV-2: Review article.Inform Med Unlocked. 2021;24:100604. doi: 10.1016/j.imu.2021.100604. Epub 2021 May 19. Inform Med Unlocked. 2021. PMID: 34028468 Free PMC article. Review.
-
COVID-19: Potential Repurposing Drugs.Infect Disord Drug Targets. 2022;22(1):e110122191924. doi: 10.2174/1871526521666210301143441. Infect Disord Drug Targets. 2022. PMID: 33645490 Review.
References
-
- Ritchie H.; et al.Coronavirus Pandemic (COVID-19) (2020–2022). Our World in Data. https://ourworldindata.org/coronavirus (accessed 2024-07-24).
- WHO COVID-19 dashboard. World Health Organization (WHO). https://data.who.int/dashboards/covid19/cases.
-
- COVID-19 Epidemiological Update – 17 May 2024. WHO. https://www.who.int/publications/m/item/covid-19-epidemiological-update-....
-
- Novel Coronavirus (2019-nCoV) Situation Reports-22. WHO. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2... (accessed 2020-02-12).
-
- Notice of the National Health Commission of the People’s Republic of China on revising the English name of novel coronavirus pneumonia (in Chinese). National Health Commission of the People’s Republic of China, 2020. http://www.nhc.gov.cn/yzygj/s7653p/202002/33393aa53d984ccdb1053a52b6bef8... (accessed 2020-02-29).
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
