Modelling the potential clinical and economic impact of universal immunisation with nirsevimab versus standard of practice for protecting all neonates and infants in their first respiratory syncytial virus season in Spain
- PMID: 39242545
- PMCID: PMC11378427
- DOI: 10.1186/s12879-024-09642-0
Modelling the potential clinical and economic impact of universal immunisation with nirsevimab versus standard of practice for protecting all neonates and infants in their first respiratory syncytial virus season in Spain
Abstract
Background: Respiratory syncytial virus (RSV) is associated with substantial morbidity among infants. This study modelled the potential public health and economic impact of nirsevimab, a long-acting monoclonal antibody, as an immunoprophylactic strategy for all infants in Spain in their first RSV season.
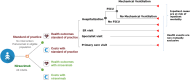
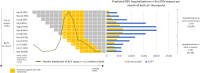
Methods: A static decision-analytic model of the Spanish birth cohort during its first RSV season was developed to estimate the impact of nirsevimab on RSV-related health events and costs versus the standard of practice (SoP). Spain-specific costs and epidemiological data were used as model inputs. Modelled outcomes included RSV-related outpatient visits, emerging room (ER) visits, hospitalisations - including pediatric intensive care unit (PICU) admission, mechanical ventilation, and inpatient mortality.
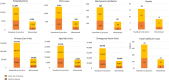
Results: Under the current SoP, RSV caused 151,741 primary care visits, 38,798 ER visits, 12,889 hospitalisations, 1,412 PICU admissions, and 16 deaths over a single season, representing a cost of €71.8 million from a healthcare payer perspective. Universal immunisation of all infants with nirsevimab was expected to prevent 97,157 primary care visits (64.0% reduction), 24,789 ER visits (63.9%), 8,185 hospitalisations (63.5%), 869 PICU admissions (61.5%), and 9 inpatient deaths (52.6%), saving €47.8 million (62.4%) in healthcare costs.
Conclusions: These results suggest that immunisation with nirsevimab of all infants experiencing their first RSV season in Spain is likely to prevent thousands of RSV-related health events and save considerable costs versus the current SoP.
Keywords: Hospitalisation; Immunisation; Infant; Nirsevimab; Public health; Respiratory syncytial viruses; Respiratory tract infections; Spain.
© 2024. The Author(s).
Conflict of interest statement
RGP reports grants/honorarium from Sanofi, Merck, Pfizer, Moderna, Seqirus and GSK. JJP reports grants/personal fees from Sanofi. GD, AK, JLLB, and MB are employees of Sanofi and may hold shares and/or stock options in the company. JR, PK, AS, and SdB are employed by Evidera, a part of Thermo Fisher Scientific that receives funding for research from Sanofi. JAA reports grants/honorarium from Sanofi, Merck, Pfizer, GSK, and AstraZeneca.
Figures

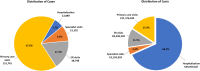

Similar articles
-
Respiratory Syncytial Virus (RSV) Burden in Infants in the Kingdom of Saudi Arabia and the Impact of All-Infant RSV Protection: A Modeling Study.Adv Ther. 2024 Apr;41(4):1419-1435. doi: 10.1007/s12325-024-02798-w. Epub 2024 Feb 15. Adv Ther. 2024. PMID: 38356106 Free PMC article.
-
Expected Impact of Universal Immunization With Nirsevimab Against RSV-Related Outcomes and Costs Among All US Infants in Their First RSV Season: A Static Model.J Infect Dis. 2022 Aug 15;226(Suppl 2):S282-S292. doi: 10.1093/infdis/jiac216. J Infect Dis. 2022. PMID: 35968866 Free PMC article.
-
Introduction of nirsevimab in Catalonia, Spain: description of the incidence of bronchiolitis and respiratory syncytial virus in the 2023/2024 season.Eur J Pediatr. 2024 Dec;183(12):5181-5189. doi: 10.1007/s00431-024-05779-x. Epub 2024 Sep 28. Eur J Pediatr. 2024. PMID: 39340677 Free PMC article.
-
Nirsevimab: Alleviating the burden of RSV morbidity in young children.J Paediatr Child Health. 2024 Oct;60(10):489-498. doi: 10.1111/jpc.16643. Epub 2024 Aug 16. J Paediatr Child Health. 2024. PMID: 39150043 Review.
-
First real-world data on universal respiratory syncytial virus prophylaxis with Nirsevimab in infants.J Prev Med Hyg. 2024 Aug 31;65(2):E172-E187. doi: 10.15167/2421-4248/jpmh2024.65.2.3329. eCollection 2024 Jun. J Prev Med Hyg. 2024. PMID: 39430977 Free PMC article. Review.
References
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140(6):543–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

