Ion transport and ultra-efficient osmotic power generation in boron nitride nanotube porins
- PMID: 39241077
- PMCID: PMC11378945
- DOI: 10.1126/sciadv.ado8081
Ion transport and ultra-efficient osmotic power generation in boron nitride nanotube porins
Abstract
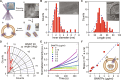
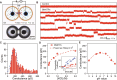
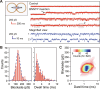
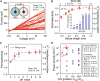
Nanotube porins form transmembrane nanomaterial-derived scaffolds that mimic the geometry and functionality of biological membrane channels. We report synthesis, transport properties, and osmotic energy harvesting performance of another member of the nanotube porin family: boron nitride nanotube porins (BNNTPs). Cryo-transmission electron microscopy imaging, liposome transport assays, and DNA translocation experiments show that BNNTPs reconstitute into lipid membranes to form functional channels of ~2-nm diameter. Ion transport studies reveal ion conductance characteristics of individual BNNTPs, which show an unusual C1/4 scaling with ion concentration and pronounced pH sensitivity. Reversal potential measurements indicate that BNNTPs have strong cation selectivity at neutral pH, attributable to the high negative charge on the channel. BNNTPs also deliver very large power density up to 12 kW/m2 in the osmotic gradient transport experiments at neutral pH, surpassing that of other BNNT-based devices by two orders of magnitude under similar conditions. Our results suggest that BNNTPs are a promising platform for mass transport and osmotic power generation.
Figures
Similar articles
-
Strong Electroosmotic Coupling Dominates Ion Conductance of 1.5 nm Diameter Carbon Nanotube Porins.ACS Nano. 2019 Nov 26;13(11):12851-12859. doi: 10.1021/acsnano.9b05118. Epub 2019 Nov 7. ACS Nano. 2019. PMID: 31682401
-
Giant osmotic energy conversion measured in a single transmembrane boron nitride nanotube.Nature. 2013 Feb 28;494(7438):455-8. doi: 10.1038/nature11876. Nature. 2013. PMID: 23446417
-
Single-Layer Hexagonal Boron Nitride Nanopores as High-Performance Ionic Gradient Power Generators.Small. 2024 Apr;20(16):e2306018. doi: 10.1002/smll.202306018. Epub 2023 Dec 2. Small. 2024. PMID: 38041449
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Pores from mitochondrial outer membranes of yeast and a porin-deficient yeast mutant: a comparison.J Bioenerg Biomembr. 1989 Aug;21(4):439-50. doi: 10.1007/BF00762516. J Bioenerg Biomembr. 1989. PMID: 2478530 Review.
References
-
- Howorka S., Building membrane nanopores. Nat. Nanotechnol. 12, 619–630 (2017). - PubMed
-
- Flood E., Boiteux C., Lev B., Vorobyov I., Allen T. W., Atomistic simulations of membrane ion channel conduction, gating, and modulation. Chem. Rev. 119, 7737–7832 (2019). - PubMed
-
- Zhang H., Li X., Hou J., Jiang L., Wang H., Angstrom-scale ion channels towards single-ion selectivity. Chem. Soc. Rev. 51, 2224–2254 (2022). - PubMed
-
- Zhang Z., Wen L., Jiang L., Bioinspired smart asymmetric nanochannel membranes. Chem. Soc. Rev. 47, 322–356 (2018). - PubMed

