GALNT9 enrichment attenuates MPP+-induced cytotoxicity by ameliorating protein aggregations containing α-synuclein and mitochondrial dysfunction
- PMID: 39237967
- PMCID: PMC11378468
- DOI: 10.1186/s13062-024-00524-8
GALNT9 enrichment attenuates MPP+-induced cytotoxicity by ameliorating protein aggregations containing α-synuclein and mitochondrial dysfunction
Abstract
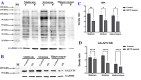
Background: GALNTs (UDP-GalNAc; polypeptide N-acetylgalactosaminyltransferases) initiate mucin-type O-GalNAc glycosylation by adding N-GalNAc to protein serine/threonine residues. Abnormalities in O-GalNAc glycosylation are involved in various disorders such as Parkinson's disease (PD), a neurodegenerative disorder. GALNT9 is potentially downregulated in PD patients.
Methods: To determine whether GALNT9 enrichment ameliorates cytotoxicity related to PD-like variations, a pcDNA3.1-GALNT9 plasmid was constructed and transfected into SH-SY5Y cells to establish a GALNT9-overexpressing cell model.
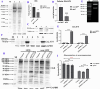
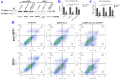
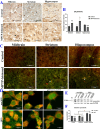
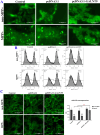
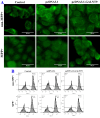
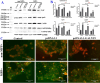
Results: Downregulation of GALNT9 and O-GalNAc glycosylation was confirmed in our animal and cellular models of PD-like variations. GALNT9 supplementation greatly attenuated cytotoxicity induced by MPP+ (1-Methyl-4-phenylpyridinium iodide) since it led to increased levels of tyrosine hydroxylase and dopamine, reduced rates of apoptosis, and significantly ameliorated MPP+-induced mitochondrial dysfunction by alleviating abnormal levels of mitochondrial membrane potential and reactive oxygen species. A long-lasting mPTP (mitochondrial permeability transition pores) opening and calcium efflux resulted in significantly lower activity in the cytochrome C-associated apoptotic pathway and mitophagy process, signifying that GALNT9 supplementation maintained neuronal cell health under MPP+ exposure. Additionally, it was found that glycans linked to proteins influenced the formation of protein aggregates containing α-synuclein, and GALNT9 supplement dramatically reduced such insoluble protein aggregations under MPP+ treatment. Glial GALNT9 predominantly appears under pathological conditions like PD-like variations.
Conclusions: GALNT9 enrichment improved cell survival, and glial GALNT9 potentially represents a pathogenic index for PD patients. This study provides insights into the development of therapeutic strategies for the treatment of PD.
Keywords: GALNT; Glycosylation; Mitochondrial dysfunction; Parkinson’s disease; Α-synuclein.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Deficiency of polypeptide N-acetylgalactosamine transferase 9 contributes to a risk for Parkinson's disease via mitochondrial dysfunctions.Int J Biol Macromol. 2024 Apr;263(Pt 2):130347. doi: 10.1016/j.ijbiomac.2024.130347. Epub 2024 Feb 23. Int J Biol Macromol. 2024. PMID: 38401583
-
MiR-29a efficiently suppresses the generation of reactive oxygen species and α-synuclein in a cellular model of Parkinson's disease by potentially targeting GSK-3β.Eur J Pharmacol. 2024 Jul 5;974:176615. doi: 10.1016/j.ejphar.2024.176615. Epub 2024 Apr 27. Eur J Pharmacol. 2024. PMID: 38685306
-
Long non-coding RNA Opa interacting protein 5-antisense RNA 1 promotes mitochondrial autophagy and protects SH-SY5Y cells from 1-methyl-4-phenylpyridine-induced damage by binding to microRNA-137 and upregulating NIX.Kaohsiung J Med Sci. 2022 Mar;38(3):207-217. doi: 10.1002/kjm2.12485. Epub 2022 Jan 20. Kaohsiung J Med Sci. 2022. PMID: 35049152
-
Reprint of: revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease-resemblance to the effect of amphetamine drugs of abuse.Free Radic Biol Med. 2013 Sep;62:186-201. doi: 10.1016/j.freeradbiomed.2013.05.042. Epub 2013 Jun 3. Free Radic Biol Med. 2013. PMID: 23743292 Review.
-
Polypeptide N-acetylgalactosaminyltransferase-Associated Phenotypes in Mammals.Molecules. 2021 Sep 10;26(18):5504. doi: 10.3390/molecules26185504. Molecules. 2021. PMID: 34576978 Free PMC article. Review.
Cited by
-
Calcium signaling regulates the accumulation of phenolic acids in response to UV-B stress in Rhododendron chrysanthum Pall.Plant Cell Rep. 2024 Aug 31;43(9):224. doi: 10.1007/s00299-024-03308-6. Plant Cell Rep. 2024. PMID: 39215829
References
-
- Brockhausen I, Stanley P. O-GalNAc glycans–essentials of glycobiology. Cold Spring Harbor (NY):Cold Spring Harbor Lab Press. 2017;3rd edition:Chap10.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

