PARVB deficiency alleviates cisplatin-induced tubular injury by inhibiting TAK1 signaling
- PMID: 39235496
- PMCID: PMC11377400
- DOI: 10.1007/s00018-024-05422-w
PARVB deficiency alleviates cisplatin-induced tubular injury by inhibiting TAK1 signaling
Abstract
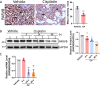
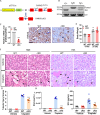
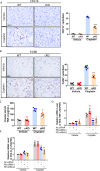
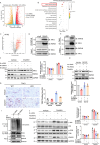
Cisplatin-induced renal tubular injury largely restricts the wide-spread usage of cisplatin in the treatment of malignancies. Identifying the key signaling pathways that regulate cisplatin-induced renal tubular injury is thus clinically important. PARVB, a focal adhesion protein, plays a crucial role in tumorigenesis. However, the function of PARVB in kidney disease is largely unknown. To investigate whether and how PARVB contributes to cisplatin-induced renal tubular injury, a mouse model (PARVB cKO) was generated in which PARVB gene was specifically deleted from proximal tubular epithelial cells using the Cre-LoxP system. In this study, we found depletion of PARVB in proximal tubular epithelial cells significantly attenuates cisplatin-induced renal tubular injury, including tubular cell death and inflammation. Mechanistically, PARVB associates with transforming growth factor-β-activated kinase 1 (TAK1), a central regulator of cell survival and inflammation that is critically involved in mediating cisplatin-induced renal tubular injury. Depletion of PARVB promotes cisplatin-induced TAK1 degradation, inhibits TAK1 downstream signaling, and ultimately alleviates cisplatin-induced tubular cell damage. Restoration of PARVB or TAK1 in PARVB-deficient cells aggravates cisplatin-induced tubular cell injury. Finally, we demonstrated that PARVB regulates TAK1 protein expression through an E3 ligase ITCH-dependent pathway. PARVB prevents ITCH association with TAK1 to block its ubiquitination. Our study reveals that PARVB deficiency protects against cisplatin-induced tubular injury through regulation of TAK1 signaling and indicates targeting this pathway may provide a novel therapeutic strategy to alleviate cisplatin-induced kidney damage.
Keywords: Beta-parvin; Cell death; Focal adhesion; ITCH; Inflammation; Kidney diseases.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
TAK1 deficiency attenuates cisplatin-induced acute kidney injury.Am J Physiol Renal Physiol. 2020 Jan 1;318(1):F209-F215. doi: 10.1152/ajprenal.00516.2019. Epub 2019 Dec 9. Am J Physiol Renal Physiol. 2020. PMID: 31813254 Free PMC article.
-
Dual-specificity phosphatase 26 protects against kidney injury caused by ischaemia-reperfusion through restraint of TAK1-JNK/p38-mediated apoptosis and inflammation of renal tubular epithelial cells.Toxicol Appl Pharmacol. 2024 Jun;487:116954. doi: 10.1016/j.taap.2024.116954. Epub 2024 May 4. Toxicol Appl Pharmacol. 2024. PMID: 38705402
-
TAK1 mediates excessive autophagy via p38 and ERK in cisplatin-induced acute kidney injury.J Cell Mol Med. 2018 May;22(5):2908-2921. doi: 10.1111/jcmm.13585. Epub 2018 Mar 5. J Cell Mol Med. 2018. PMID: 29504713 Free PMC article.
-
3-deazaneplanocin A protects against cisplatin-induced renal tubular cell apoptosis and acute kidney injury by restoration of E-cadherin expression.Cell Death Dis. 2019 May 1;10(5):355. doi: 10.1038/s41419-019-1589-y. Cell Death Dis. 2019. PMID: 31043583 Free PMC article.
-
Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity.Autophagy. 2008 Jul;4(5):710-2. doi: 10.4161/auto.6309. Epub 2008 May 20. Autophagy. 2008. PMID: 18497570 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

