Clearing the JUNQ: the molecular machinery for sequestration, localization, and degradation of the JUNQ compartment
- PMID: 39234568
- PMCID: PMC11372896
- DOI: 10.3389/fmolb.2024.1427542
Clearing the JUNQ: the molecular machinery for sequestration, localization, and degradation of the JUNQ compartment
Abstract
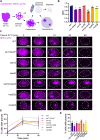
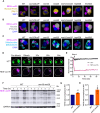
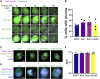
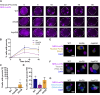
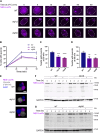
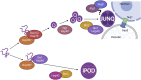
Cellular protein homeostasis (proteostasis) plays an essential role in regulating the folding, sequestration, and turnover of misfolded proteins via a network of chaperones and clearance factors. Previous work has shown that misfolded proteins are spatially sequestered into membrane-less compartments in the cell as part of the proteostasis process. Soluble misfolded proteins in the cytoplasm are trafficked into the juxtanuclear quality control compartment (JUNQ), and nuclear proteins are sequestered into the intranuclear quality control compartment (INQ). However, the mechanisms that control the formation, localization, and degradation of these compartments are unknown. Previously, we showed that the JUNQ migrates to the nuclear membrane adjacent to the INQ at nucleus-vacuole junctions (NVJ), and the INQ moves through the NVJ into the vacuole for clearance in an ESCRT-mediated process. Here we have investigated what mechanisms are involved in the formation, migration, and clearance of the JUNQ. We find Hsp70s Ssa1 and Ssa2 are required for JUNQ localization to the NVJ and degradation of cytoplasmic misfolded proteins. We also confirm that sequestrases Btn2 and Hsp42 sort misfolded proteins to the JUNQ or IPOD, respectively. Interestingly, proteins required for piecemeal microautophagy of the nucleus (PMN) (i.e., Nvj1, Vac8, Atg1, and Atg8) drive the formation and clearance of the JUNQ. This suggests that the JUNQ migrates to the NVJ to be cleared via microautophagy.
Keywords: microautophagy; piecemeal microautophagy of the nucleus; protein misfolding; proteostasis; spatial sequestration.
Copyright © 2024 Rolli, Langridge and Sontag.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Compartment-specific aggregases direct distinct nuclear and cytoplasmic aggregate deposition.EMBO J. 2015 Mar 12;34(6):778-97. doi: 10.15252/embj.201489524. Epub 2015 Feb 11. EMBO J. 2015. PMID: 25672362 Free PMC article.
-
Quaternary structures of Vac8 differentially regulate the Cvt and PMN pathways.Autophagy. 2020 Jun;16(6):991-1006. doi: 10.1080/15548627.2019.1659615. Epub 2019 Sep 12. Autophagy. 2020. PMID: 31512555 Free PMC article.
-
Spatially organized aggregation of misfolded proteins as cellular stress defense strategy.J Mol Biol. 2015 Apr 10;427(7):1564-74. doi: 10.1016/j.jmb.2015.02.006. Epub 2015 Feb 11. J Mol Biol. 2015. PMID: 25681695 Review.
-
Nuclear and cytoplasmic spatial protein quality control is coordinated by nuclear-vacuolar junctions and perinuclear ESCRT.Nat Cell Biol. 2023 May;25(5):699-713. doi: 10.1038/s41556-023-01128-6. Epub 2023 Apr 20. Nat Cell Biol. 2023. PMID: 37081164
-
Proteotoxicity: A Fatal Consequence of Environmental Pollutants-Induced Impairments in Protein Clearance Machinery.J Pers Med. 2021 Jan 25;11(2):69. doi: 10.3390/jpm11020069. J Pers Med. 2021. PMID: 33503824 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

