Proteomics analysis of periplaque and chronic inactive multiple sclerosis lesions
- PMID: 39234409
- PMCID: PMC11371774
- DOI: 10.3389/fnmol.2024.1448215
Proteomics analysis of periplaque and chronic inactive multiple sclerosis lesions
Abstract
Background: Multiple sclerosis (MS) is a demyelinating disease of the central nervous system characterized by increased inflammation and immune responses, oxidative injury, mitochondrial dysfunction, and iron dyshomeostasis leading to demyelination and axonal damage. In MS, incomplete remyelination results in chronically demyelinated axons and degeneration coinciding with disability. This suggests a failure in the ability to remyelinate in MS, however, the precise underlying mechanisms remain unclear. We aimed to identify proteins whose expression was altered in chronic inactive white matter lesions and periplaque white matter in MS tissue to reveal potential pathophysiological mechanisms.
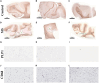
Methods: Laser capture microdissection coupled to proteomics was used to interrogate spatially altered changes in formalin-fixed paraffin-embedded brain tissue from three chronic MS individuals and three controls with no apparent neurological complications. Histopathological maps guided the capture of inactive lesions, periplaque white matter, and cortex from chronic MS individuals along with corresponding white matter and cortex from control tissue. Label free quantitation by liquid chromatography tandem mass spectrometry was used to discover differentially expressed proteins between the various brain regions.
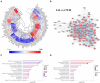
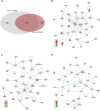
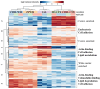
Results: In addition to confirming loss of several myelin-associated proteins known to be affected in MS, proteomics analysis of chronic inactive MS lesions revealed alterations in myelin assembly, metabolism, and cytoskeletal organization. The top altered proteins in MS inactive lesions compared to control white matter consisted of PPP1R14A, ERMN, SIRT2, CARNS1, and MBLAC2.
Conclusion: Our findings highlight proteome changes in chronic inactive MS white matter lesions and periplaque white matter, which may be crucial for proper myelinogenesis, bioenergetics, focal adhesions, and cellular function. This study highlights the importance and feasibility of spatial approaches such as laser capture microdissection-based proteomics analysis of pathologically distinct regions of MS brain tissue. Identification of spatially resolved changes in the proteome of MS brain tissue should aid in the understanding of pathophysiological mechanisms and the development of novel therapies.
Keywords: differentially expressed proteins; multiple sclerosis; pathology; protein networks; proteomics; spatial profiling.
Copyright © 2024 Wilkins, Mangalaparthi, Netzel, Sherman, Guo, Kalinowska-Lyszczarz, Pandey and Lucchinetti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Relationship of acute axonal damage, Wallerian degeneration, and clinical disability in multiple sclerosis.J Neuroinflammation. 2017 Mar 17;14(1):57. doi: 10.1186/s12974-017-0831-8. J Neuroinflammation. 2017. PMID: 28302146 Free PMC article.
-
Expansion of chronic MS lesions is associated with an increase of radial diffusivity in periplaque white matter.Mult Scler. 2022 Apr;28(5):697-706. doi: 10.1177/13524585211033464. Epub 2021 Aug 11. Mult Scler. 2022. PMID: 34378454
-
Comprehensive tissue processing strategy for quantitative proteomics of formalin-fixed multiple sclerosis lesions.J Proteome Res. 2011 Oct 7;10(10):4855-68. doi: 10.1021/pr200672n. Epub 2011 Sep 14. J Proteome Res. 2011. PMID: 21870854
-
Chronic Demyelination and Axonal Degeneration in Multiple Sclerosis: Pathogenesis and Therapeutic Implications.Curr Neurol Neurosci Rep. 2021 Apr 9;21(6):26. doi: 10.1007/s11910-021-01110-5. Curr Neurol Neurosci Rep. 2021. PMID: 33835275 Review.
-
Remodeling of the interstitial extracellular matrix in white matter multiple sclerosis lesions: Implications for remyelination (failure).J Neurosci Res. 2020 Jul;98(7):1370-1397. doi: 10.1002/jnr.24582. Epub 2020 Jan 21. J Neurosci Res. 2020. PMID: 31965607 Review.
References
-
- Bramow S., Frischer J., Lassmann H., Koch-Henriksen N., Lucchinetti C., Sørensen P., et al. (2010). Demyelination versus remyelination in progressive multiple sclerosis. Brain 133 2983–2998. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

