Transcriptome analysis of the aged SAMP8 mouse model of Alzheimer's disease reveals novel molecular targets of formononetin protection
- PMID: 39234102
- PMCID: PMC11371586
- DOI: 10.3389/fphar.2024.1440515
Transcriptome analysis of the aged SAMP8 mouse model of Alzheimer's disease reveals novel molecular targets of formononetin protection
Abstract
Background: Senescence-accelerated mouse prone 8 (SAMP8) and age-matched SAMR1 mice are used to study the pathogenesis and therapeutics of Alzheimer's disease (AD); however, the molecular mechanisms are not completely understood.
Objective: This study aimed to examine the effects of the 5-month administration of formononetin in SAMP8 mice and used RNA-seq to explore the molecular targets.
Methods: SAMP8 mice were orally administered formononetin (0, 8, and 16 mg/kg) from 4 months of age, and age-matched SAMR1 mice were used as controls. Behavioral tests were performed in 9-month-old mice, followed by histopathologic analysis. Total RNA from the hippocampus was isolated and subjected to RNA-seq, RT-qPCR, and bioinformatics analysis.
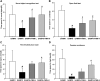
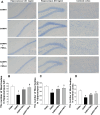
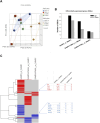
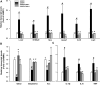
Results: The 9-month-old SAMP8 mice exhibited cognition deficits, evidenced by novel object recognition, open-field test, elevated plus maze, and passive avoidance. Nissl bodies in the cortex and hippocampus were decreased. Formononetin treatments ameliorated behavioral deficits and improved morphological changes, which were evidenced by Nissl and H&E staining. RNA-seq revealed distinct gene expression patterns between SAMP8 and SAMR1 mice. Differentially expressed genes in SAMP8 mice were attenuated or normalized by formononetin. Ingenuity pathway analysis (IPA) of canonical pathway and upstream regulators revealed increases in proinflammatory factors and immune dysfunction and decreases in NRF2 and SIRT-1 signaling pathways, leading to neuroinflammation. Formononetin treatment attenuated or reversed these molecular changes. The transcriptome of SAMP8 mice was correlated with transcriptomic profiles of other AD mouse models in the GEO database.
Conclusion: Neuroinflammation and decreased antioxidant and SIRT-1 signaling contributed to cognitive deficits in aged SAMP8 mice, which are potential therapeutic targets of formononetin in combination with other therapies.
Keywords: Alzheimer’s disease; RNA-seq analysis; aged SAMP8 mouse; formononetin; ingenuity pathway analysis.
Copyright © 2024 Liu, Cui, Liu and Shi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Proteomic analysis of anti-aging effects of Dendrobium nobile Lindl. alkaloids in aging-accelerated SAMP8 mice.Exp Gerontol. 2023 Jun 15;177:112198. doi: 10.1016/j.exger.2023.112198. Epub 2023 May 6. Exp Gerontol. 2023. PMID: 37150330
-
Multi-strain probiotics ameliorate Alzheimer's-like cognitive impairment and pathological changes through the AKT/GSK-3β pathway in senescence-accelerated mouse prone 8 mice.Brain Behav Immun. 2024 Jul;119:14-27. doi: 10.1016/j.bbi.2024.03.031. Epub 2024 Mar 26. Brain Behav Immun. 2024. PMID: 38548184
-
The Effects of LW-AFC on the Hippocampal Transcriptome in Senescence-Accelerated Mouse Prone 8 Strain, a Mouse Model of Alzheimer's Disease.J Alzheimers Dis. 2017;57(1):227-240. doi: 10.3233/JAD-161079. J Alzheimers Dis. 2017. PMID: 28222521
-
Dendrobium nobile Lindl. Alkaloids Ameliorate Cognitive Dysfunction in Senescence Accelerated SAMP8 Mice by Decreasing Amyloid-β Aggregation and Enhancing Autophagy Activity.J Alzheimers Dis. 2020;76(2):657-669. doi: 10.3233/JAD-200308. J Alzheimers Dis. 2020. PMID: 32538851
-
Downregulated Nuclear Factor E2-Related Factor 2 (Nrf2) Aggravates Cognitive Impairments via Neuroinflammation and Synaptic Plasticity in the Senescence-Accelerated Mouse Prone 8 (SAMP8) Mouse: A Model of Accelerated Senescence.Med Sci Monit. 2018 Feb 23;24:1132-1144. doi: 10.12659/msm.908954. Med Sci Monit. 2018. PMID: 29474348 Free PMC article.
References
-
- Aboushanab S. A., Khedr S. M., Gette I. F., Danilova I. G., Kolberg N. A., Ravishankar G. A., et al. (2022). Isoflavones derived from plant raw materials: bioavailability, anti-cancer, anti-aging potentials, and microbiome modulation. Crit. Rev. Food Sci. Nutr. 63, 261–287. 10.1080/10408398.2021.1946006 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

