The usefulness of anti-HCV signal to cut-off ratio in predicting hepatitis C viremia and the effect of genotype differences on signal to cut-off ratio
- PMID: 39230144
- PMCID: PMC11370746
- DOI: 10.1590/1806-9282.20240370
The usefulness of anti-HCV signal to cut-off ratio in predicting hepatitis C viremia and the effect of genotype differences on signal to cut-off ratio
Abstract
Objective: In the hepatitis C virus (HCV) diagnostic algorithm, an anti-HCV screening test is recommended first. In countries with low HCV prevalence, anti-HCV testing can often give false-positive results. This may lead to unnecessary retesting, increased costs, and psychological stress for patients.
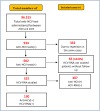
Methods: In this study, the most appropriate S/Co (signal-cutoff) value to predict HCV viremia in anti-HCV test(+) individuals was determined, and the effect of genotype differences was evaluated. Of the 96,515 anti-HCV tests performed between 2020 and 2023, 934 were reactive. A total of 332 retests and 65 patients without HCV-ribonucleic acid (RNA) analysis were excluded. Demographic data were calculated for 537 patients, and 130 patients were included in the study.
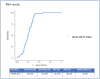
Results: The average age of 537 patients was 55±18 years, and 57.1% were women. The anti-HCV positivity rate was 0.62% (602/96,515), and the actual anti-HCV positivity rate was 0.13% (130/96,515). Anti-HCV levels were higher in HCV-RNA(+) patients than in HCV-RNA-negative individuals (p<0.0001) (Table 1). Receiver operating characteristic curve analysis identified the optimal S/Co value to be 10.86 to identify true positive cases. Sensitivity was 96.1%, specificity was 61.2%, positive predictive value (PPV) was 44.2%, and negative predictive value (NPV) was 98% (Figure 2). A total of 107 (82.3%) of the patients were identified as GT1, and the most common subtype was GT1b (n=100).
Conclusion: If anti-HCV S/Co is ≥10.86, direct HCV RNA testing may be recommended; However, the possibility of false positivity should be considered in patients with a S/Co value below 10.86.
Conflict of interest statement
Figures
Similar articles
-
[Investigation of Anti-HCV S/CO Value in Detecting Viremia in Patients with Hepatitis C Virus Infection].Mikrobiyol Bul. 2020 Jan;54(1):110-119. doi: 10.5578/mb.68833. Mikrobiyol Bul. 2020. PMID: 32050882 Turkish.
-
Significance of anti-HCV signal-to-cutoff ratio in predicting hepatitis C viremia.Korean J Intern Med. 2009 Dec;24(4):302-8. doi: 10.3904/kjim.2009.24.4.302. Epub 2009 Nov 27. Korean J Intern Med. 2009. PMID: 19949727 Free PMC article.
-
Anti-HCV antibody titer highly predicts HCV viremia in patients with hepatitis B virus dual-infection.PLoS One. 2021 Jul 1;16(7):e0254028. doi: 10.1371/journal.pone.0254028. eCollection 2021. PLoS One. 2021. PMID: 34197557 Free PMC article.
-
Utilization of Signal-to-Cutoff Ratio of Hepatitis C Virus Antibody Assay in Predicting HCV Viremia among Hemodialysis Patients.Nephron. 2015;130(2):127-33. doi: 10.1159/000430988. Epub 2015 Jun 6. Nephron. 2015. PMID: 26065912
-
Determination of the cut-off value of serum alanine aminotransferase in patients undergoing hemodialysis, to identify biochemical activity in patients with hepatitis C viremia.J Clin Virol. 2006 Mar;35(3):298-302. doi: 10.1016/j.jcv.2005.09.010. Epub 2005 Nov 11. J Clin Virol. 2006. PMID: 16290052
References
-
- World Health Organization . Updated recommendations on treatment of adolescents and children with chronic HCV infection, and HCV simplified service delivery and diagnostics. Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization; 2022. - PubMed
-
- European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu; Clinical Practice Guidelines Panel: Chair:; EASL Governing Board representative:; Panel members:. EASL recommendations on treatment of hepatitis C: Final update of the series☆ . J Hepatol. 2020;73(5):1170–1218. doi: 10.1016/j.jhep.2020.08.018. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical