This is a preprint.
MiniXL: An open-source, large field-of-view epifluorescence miniature microscope for mice capable of single-cell resolution and multi-brain region imaging
- PMID: 39229051
- PMCID: PMC11370419
- DOI: 10.1101/2024.08.16.608328
MiniXL: An open-source, large field-of-view epifluorescence miniature microscope for mice capable of single-cell resolution and multi-brain region imaging
Abstract
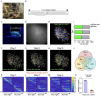
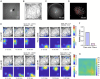
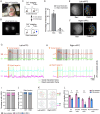
Capturing the intricate dynamics of neural activity in freely behaving animals is essential for understanding the neural mechanisms underpinning specific behaviors. Miniaturized microscopy enables investigators to track population activity at cellular level, but the field of view (FOV) of these microscopes have been limited and does not allow multiple-brain region imaging. To fill this technological gap, we have developed the eXtra Large field-of-view Miniscope (MiniXL), a 3.5g lightweight miniaturized microscope with an FOV measuring 3.5 mm in diameter and an electrically adjustable working distance of 1.9 mm ± 200 μm. We demonstrated the capability of MiniXL recording the activity of large neuronal population in both subcortical area (hippocampal dorsal CA1) and deep brain regions (medial prefrontal cortex, mPFC and nucleus accumbens, NAc). The large FOV allows simultaneous imaging of multiple brain regions such as bilateral mPFCs or mPFC and NAc during complex social behavior and tracking cells across multiple sessions. As with all microscopes in the UCLA Miniscope ecosystem, the MiniXL is fully open-source and will be shared with the neuroscience community to lower the barriers for adoption of this technology.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Miniscope-LFOV: A large-field-of-view, single-cell-resolution, miniature microscope for wired and wire-free imaging of neural dynamics in freely behaving animals.Sci Adv. 2023 Apr 21;9(16):eadg3918. doi: 10.1126/sciadv.adg3918. Epub 2023 Apr 21. Sci Adv. 2023. PMID: 37083539 Free PMC article.
-
Pan-cortical cellular imaging in freely behaving mice using a miniaturized micro-camera array microscope (mini-MCAM).bioRxiv [Preprint]. 2024 Jul 29:2024.07.04.601964. doi: 10.1101/2024.07.04.601964. bioRxiv. 2024. PMID: 39005454 Free PMC article. Preprint.
-
A wireless miniScope for deep brain imaging in freely moving mice.J Neurosci Methods. 2019 Jul 15;323:56-60. doi: 10.1016/j.jneumeth.2019.05.008. Epub 2019 May 19. J Neurosci Methods. 2019. PMID: 31116963 Free PMC article.
-
In Vivo Observations of Rapid Scattered Light Changes Associated with Neurophysiological Activity.In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 5. In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 5. PMID: 26844322 Free Books & Documents. Review.
-
New imaging instrument in animal models: Two-photon miniature microscope and large field of view miniature microscope for freely behaving animals.J Neurochem. 2023 Feb;164(3):270-283. doi: 10.1111/jnc.15711. Epub 2022 Nov 5. J Neurochem. 2023. PMID: 36281555 Review.
References
-
- Zong W., Wu R., Li M., Hu Y., Li Y., Li J., Rong H., Wu H., Xu Y., Lu Y., Jia H., Fan M., Zhou Z., Zhang Y., Wang A., Chen L., Cheng H., Fast high-resolution miniature two-photon microscopy for brain imaging in freely behaving mice. Nat Methods 14, 713–719 (2017). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
