An unscheduled switch to endocycles induces a reversible senescent arrest that impairs growth of the Drosophila wing disc
- PMID: 39226333
- PMCID: PMC11398662
- DOI: 10.1371/journal.pgen.1011387
An unscheduled switch to endocycles induces a reversible senescent arrest that impairs growth of the Drosophila wing disc
Abstract
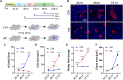
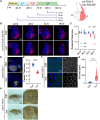
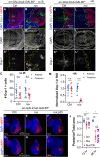
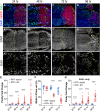
A programmed developmental switch to G / S endocycles results in tissue growth through an increase in cell size. Unscheduled, induced endocycling cells (iECs) promote wound healing but also contribute to cancer. Much remains unknown, however, about how these iECs affect tissue growth. Using the D. melanogaster wing disc as model, we find that populations of iECs initially increase in size but then subsequently undergo a heterogenous arrest that causes severe tissue undergrowth. iECs acquired DNA damage and activated a Jun N-terminal kinase (JNK) pathway, but, unlike other stressed cells, were apoptosis-resistant and not eliminated from the epithelium. Instead, iECs entered a JNK-dependent and reversible senescent-like arrest. Senescent iECs promoted division of diploid neighbors, but this compensatory proliferation did not rescue tissue growth. Our study has uncovered unique attributes of iECs and their effects on tissue growth that have important implications for understanding their roles in wound healing and cancer.
Copyright: © 2024 Huang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Update of
-
An unscheduled switch to endocycles induces a reversible senescent arrest that impairs growth of the Drosophila wing disc.bioRxiv [Preprint]. 2024 Mar 14:2024.03.14.585098. doi: 10.1101/2024.03.14.585098. bioRxiv. 2024. Update in: PLoS Genet. 2024 Sep 3;20(9):e1011387. doi: 10.1371/journal.pgen.1011387 PMID: 38559130 Free PMC article. Updated. Preprint.
Similar articles
-
An unscheduled switch to endocycles induces a reversible senescent arrest that impairs growth of the Drosophila wing disc.bioRxiv [Preprint]. 2024 Mar 14:2024.03.14.585098. doi: 10.1101/2024.03.14.585098. bioRxiv. 2024. Update in: PLoS Genet. 2024 Sep 3;20(9):e1011387. doi: 10.1371/journal.pgen.1011387 PMID: 38559130 Free PMC article. Updated. Preprint.
-
JNK-dependent cell cycle stalling in G2 promotes survival and senescence-like phenotypes in tissue stress.Elife. 2019 Feb 8;8:e41036. doi: 10.7554/eLife.41036. Elife. 2019. PMID: 30735120 Free PMC article.
-
Role of D-GADD45 in JNK-Dependent Apoptosis and Regeneration in Drosophila.Genes (Basel). 2019 May 18;10(5):378. doi: 10.3390/genes10050378. Genes (Basel). 2019. PMID: 31109086 Free PMC article.
-
Pro-apoptotic and pro-proliferation functions of the JNK pathway of Drosophila: roles in cell competition, tumorigenesis and regeneration.Open Biol. 2019 Mar 29;9(3):180256. doi: 10.1098/rsob.180256. Open Biol. 2019. PMID: 30836847 Free PMC article. Review.
-
Cultivation and Live Imaging of Drosophila Imaginal Discs.Methods Mol Biol. 2016;1478:203-213. doi: 10.1007/978-1-4939-6371-3_11. Methods Mol Biol. 2016. PMID: 27730583 Review.
Cited by
-
Oncogenic signaling in the adult Drosophila prostate-like accessory gland leads to activation of a conserved pro-tumorigenic program, in the absence of proliferation.bioRxiv [Preprint]. 2024 Jun 7:2024.05.10.593549. doi: 10.1101/2024.05.10.593549. bioRxiv. 2024. PMID: 38853988 Free PMC article. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

