Real-World Effectiveness of Mepolizumab in Severe Asthma: Results from the Multi-country, Self-controlled Nucala Effectiveness Study (NEST)
- PMID: 39215767
- PMCID: PMC11480159
- DOI: 10.1007/s12325-024-02967-x
Real-World Effectiveness of Mepolizumab in Severe Asthma: Results from the Multi-country, Self-controlled Nucala Effectiveness Study (NEST)
Abstract
Introduction: The Nucala Effectiveness Study (NEST) assessed the effectiveness of mepolizumab in patients with severe asthma (SA) in countries previously underrepresented in real-world studies.
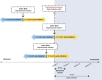
Methods: A multi-country, bi-directional, self-controlled, observational cohort study conducted in Colombia, Chile, India, Türkiye, Saudi Arabia, United Arab Emirates, Kuwait, Oman, and Qatar. Historical and/or prospective data from patients with SA were assessed 12 months pre- and post-mepolizumab initiation.
Primary endpoint: incident rate ratio (IRR) of clinically significant exacerbations (CSEs). Key secondary endpoints: healthcare resource utilisation (HCRU), oral corticosteroid (OCS) use, lung function and symptom control (Asthma Control Test [ACT] scores).
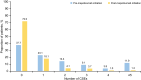
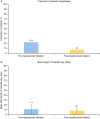
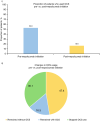
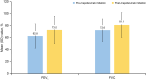
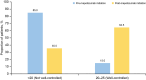
Results: Overall, 525 patients with SA burden pre-initiation (geometric mean blood eosinophil count [BEC] 490.7 cells/µl; 31.4% prior biologic use; 37.3% obese) received at least one dose of mepolizumab 100 mg subcutaneously. Post-initiation, a significant reduction in CSEs was observed (76% [p < 0.001]; IRR [95% confidence interval] 0.24 [0.19-0.30]); 72.0% of patients had no CSEs. Mepolizumab treatment led to a reduction in OCS use (52.8% pre-initiation vs. 16.6% post-initiation) and a mean (standard deviation [SD]) change in OCS dose of - 18.1 (20.7) mg post-initiation; 36.1% of patients became OCS-free. Fewer patients were hospitalised post-initiation (22.5% pre-initiation vs. 6.9% post-initiation). Improvements in mean (SD) forced expiratory volume in 1 s (62.8 [20.2]% pre-initiation vs. 73.0 [22.7]% post-initiation) and ACT scores (15.0% pre-initiation vs. 64.5% of patients post-initiation with well-controlled asthma) were observed. Proportion of patients with BEC ≥ 500 cells/µl decreased from 84.4% pre-initiation to 18.1% post-initiation.
Conclusion: Mepolizumab was effective in reducing the burden of SA by significantly reducing CSEs, reducing OCS use and HCRU, and improving lung function and asthma control, which could translate to improvements in health-related quality of life in patients with SA and high OCS dependency in the countries studied. A graphical abstract is available with this article.
Keywords: Antibodies; Asthma; Biological therapy; Corticosteroids; Interleukin-5; Low-middle-income countries; Mepolizumab; Monoclonal.
Plain language summary
Severe asthma occurs when asthma symptoms remain uncontrolled despite optimised treatment. In many low-middle income countries, and in some countries in the Middle East, Asia, Latin America and the Arab Gulf, the management and treatment of patients with severe asthma remain poor, with many patients having unscheduled hospital visits or admission, and use of steroids for a prolonged period. Mepolizumab is an injectable monoclonal antibody approved as an add-on treatment for severe asthma in patients ≥ 6 years of age. In clinical trials, mepolizumab has demonstrated reductions in the risk of clinically significant exacerbations (CSE; an asthma exacerbation that requires systemic corticosteroids and/or an emergency room visit and/or hospitalisation) and the need for oral corticosteroid (OCS) treatment in patients with severe asthma by reducing inflammation caused by eosinophil (a type of white blood cell) production. The Nucala Effectiveness Study (NEST) was performed to observe the effectiveness of mepolizumab in people with severe asthma in Colombia, Chile, India, Turkey, Saudi Arabia, United Arab Emirates, Kuwait, Oman and Qatar. The frequency of CSEs and other outcomes was compared 12 months pre- and post-mepolizumab initiation. Post-initiation, the risk of CSEs was significantly reduced by 76% (p < 0.001), and 72% of patients had no CSEs. Fewer patients were dependent on OCS, with 36.1% of patients not using OCS at all, and fewer patients were hospitalised. Lung function and asthma control also improved. NEST shows that mepolizumab could benefit people with severe asthma living in countries where disease-related burden and OCS use remain high.
© 2024. The Author(s).
Conflict of interest statement
Riyad Omar Al-Lehebi has given lectures at meetings supported by AstraZeneca, Boehringer Ingelheim, GSK and Sanofi, and received advisory board fees from GSK. Mona Al Ahmad received lecture and advisory board honoraria from Sanofi, GSK and AstraZeneca. Venkata Nagarjuna Maturu has given lectures at meetings supported by AstraZeneca, GSK, Novartis, Sun Pharma, Cipla and Lupin, and participated in advisory boards with AstraZeneca and GSK. Alejandra Galeano Mesa has no conflicts of interest to report. Bassam Mahboub is a speaker for AstraZeneca, GSK and Sanofi. Elizabeth Garcia reports having been a speaker for Sanofi, Novartis, and Pfizer and received advisory board fees from GSK, Sanofi and Novartis. Patricia Fernandez reports having been a speaker for AstraZeneca, GSK, Sanofi and Teva, and participated in advisory boards with AstraZeneca, GSK and Sanofi. Claudia Soares, Alejandro Raimondi, Maria Laucho-Contreras, Saeed Noibi and Gur Levy are employed by GSK and hold financial equities in GSK. Gabriela Abreu and Juliana Queiroz are complementary employees of GSK and do not hold financial equities in GSK. Debora dos Santos was a complementary worker of GSK at the time of the study analysis. Debora dos Santos’ current affiliation is a postdoctoral fellowship at University of Brasilia under the Program of Collective Health. Sevim Bavbek has given lectures at meetings supported by AstraZeneca, GSK and Novartis, and reports receipt of advisory board fees from AstraZeneca, GSK and Novartis.
Figures
Similar articles
-
REal worlD Effectiveness and Safety of Mepolizumab in a Multicentric Spanish Cohort of Asthma Patients Stratified by Eosinophils: The REDES Study.Drugs. 2021 Oct;81(15):1763-1774. doi: 10.1007/s40265-021-01597-9. Epub 2021 Sep 29. Drugs. 2021. PMID: 34586602 Free PMC article. Clinical Trial.
-
Mepolizumab for Treating Severe Eosinophilic Asthma: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2018 Feb;36(2):131-144. doi: 10.1007/s40273-017-0571-8. Pharmacoeconomics. 2018. PMID: 28933002 Review.
-
The inflammatory profile of exacerbations in patients with severe refractory eosinophilic asthma receiving mepolizumab (the MEX study): a prospective observational study.Lancet Respir Med. 2021 Oct;9(10):1174-1184. doi: 10.1016/S2213-2600(21)00004-7. Epub 2021 May 7. Lancet Respir Med. 2021. PMID: 33971168
-
Long-term Safety and Clinical Benefit of Mepolizumab in Patients With the Most Severe Eosinophilic Asthma: The COSMEX Study.Clin Ther. 2019 Oct;41(10):2041-2056.e5. doi: 10.1016/j.clinthera.2019.07.007. Epub 2019 Aug 22. Clin Ther. 2019. PMID: 31447130 Clinical Trial.
-
Omalizumab as alternative to chronic use of oral corticosteroids in severe asthma.Respir Med. 2019 Apr;150:51-62. doi: 10.1016/j.rmed.2019.02.003. Epub 2019 Feb 7. Respir Med. 2019. PMID: 30961951 Review.
References
-
- Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: Data from the international severe asthma registry. Chest. 2020;157(4):790–804. - PubMed
-
- Global Initiative for Asthma (GINA). 2023 gina report, global strategy for asthma management and prevention. https://ginasthma.org/2023-gina-main-report/. Accessed 1 Mar 2024.
-
- Al-Ahmad M, Al Zaabi A, Madkour A, et al. Expert consensus on oral corticosteroids stewardship for the treatment of severe asthma in the Middle East and Africa. Respir Med. 2024;228: 107674. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

