Chemoenzymatic Synthesis of Sulfated N-Glycans Recognized by Siglecs and Other Glycan-Binding Proteins
- PMID: 39211606
- PMCID: PMC11350573
- DOI: 10.1021/jacsau.4c00307
Chemoenzymatic Synthesis of Sulfated N-Glycans Recognized by Siglecs and Other Glycan-Binding Proteins
Abstract
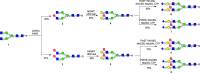
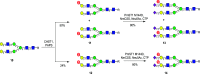
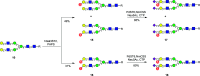
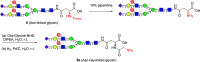
Sulfated N-glycans are present in many glycoproteins, which are implicated in playing important roles in biological recognition processes. Here, we report the systematic chemoenzymatic synthesis of a library of sulfated and sialylated biantennary N-glycans and assess their binding to Siglecs and glycan-specific antibodies that recognize them as glycan ligands. The combined use of three human sulfotransferases, GlcNAc-6-O-sulfotransferase (CHST2), Gal-3-O-sulfotransferase (Gal3ST1), and keratan sulfate Gal-6-O-sulfotransferase (CHST1), resulted in asymmetric and symmetric branch-selective sulfation of the GlcNAc and/or Gal moieties of N-glycans. The extension of the sugar chain using α-2,3- and α-2,6-sialyltransferases afforded the sulfated and sialylated N-glycans. These synthetic glycans with different patterns of sulfation and sialylation were evaluated for binding to selected Siglecs and sulfoglycan-specific antibodies using glycan microarrays. The results confirm previously documented glycan-recognizing properties and further reveal novel specificities for these glycan-binding proteins, demonstrating the utility of the library for assessing the specificity of glycan-binding proteins recognizing sulfated and sialylated glycans.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Site-selective sulfation of N-glycans by human GlcNAc-6-O-sulfotransferase 1 (CHST2) and chemoenzymatic synthesis of sulfated antibody glycoforms.Bioorg Chem. 2022 Nov;128:106070. doi: 10.1016/j.bioorg.2022.106070. Epub 2022 Aug 1. Bioorg Chem. 2022. PMID: 35939855 Free PMC article.
-
Installation of O-glycan sulfation capacities in human HEK293 cells for display of sulfated mucins.J Biol Chem. 2022 Feb;298(2):101382. doi: 10.1016/j.jbc.2021.101382. Epub 2021 Dec 24. J Biol Chem. 2022. PMID: 34954141 Free PMC article.
-
Exploiting Substrate Specificities of 6-O-Sulfotransferases to Enzymatically Synthesize Keratan Sulfate Oligosaccharides.JACS Au. 2023 Oct 13;3(11):3155-3164. doi: 10.1021/jacsau.3c00488. eCollection 2023 Nov 27. JACS Au. 2023. PMID: 38034954 Free PMC article.
-
Sialylation of N-glycans: mechanism, cellular compartmentalization and function.Histochem Cell Biol. 2017 Feb;147(2):149-174. doi: 10.1007/s00418-016-1520-x. Epub 2016 Dec 14. Histochem Cell Biol. 2017. PMID: 27975143 Free PMC article. Review.
-
Glycoproteins from insect cells: sialylated or not?Biol Chem. 2001 Feb;382(2):151-9. doi: 10.1515/BC.2001.023. Biol Chem. 2001. PMID: 11308014 Free PMC article. Review.
References
-
- Barboza M.; Duschak V. G.; Fukuyama Y.; Nonami H.; Erra-Balsells R.; Cazzulo J. J.; Couto A. S. Structural analysis of the N-glycans of the major cysteine proteinase of Trypanosoma cruzi. Identification of sulfated high-mannose type oligosaccharides. FEBS J. 2005, 272 (15), 3803–3815. 10.1111/j.1742-4658.2005.04787.x. - DOI - PubMed
-
- Baenziger J. U.; Green E. D. Pituitary glycoprotein hormone oligosaccharides: structure, synthesis and function of the asparagine-linked oligosaccharides on lutropin, follitropin and thyrotropin. Biochim. Biophys. Acta, Rev. Biomembr. 1988, 947 (2), 287–306. 10.1016/0304-4157(88)90012-3. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
