Design and validation of novel flow cytometry panels to analyze a comprehensive range of peripheral immune cells in mice
- PMID: 39206202
- PMCID: PMC11350558
- DOI: 10.3389/fimmu.2024.1432816
Design and validation of novel flow cytometry panels to analyze a comprehensive range of peripheral immune cells in mice
Abstract
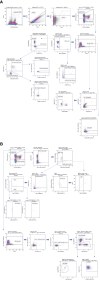
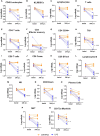
The use of flow cytometry in mice is constrained by several factors, including the limited availability of mouse-specific antibodies and the need to work with small volumes of peripheral blood. This is particularly challenging for longitudinal studies, as serial blood samples should not exceed 10% of the total blood volume in mice. To address this, we have developed two novel flow cytometry panels designed to extensively analyze immune cell populations in mice during longitudinal studies, using only 50 µL of peripheral blood per panel. Additionally, a third panel has been designed to conduct a more detailed analysis of cytotoxic and inhibitory markers at the end point. These panels have been validated on a lipopolysaccharide (LPS)-induced lung inflammation model. Two experiments were conducted to 1) validate the panels' sensitivity to immune challenges (n=12) and 2) to assess intrinsic variability of measurements (n=5). In both experiments, we collected 50 µL of peripheral blood for each cytometry panel from the maxillary venous sinus. All antibodies were titrated to identify the optimal concentration that maximized the signal from the positive population while minimizing the signal from the negative population. Samples were processed within 1 hour of collection using a MACSQuant Analyzer 16 cytometer. Our results demonstrate that these immunological panels are sensitive enough to detect changes in peripheral blood after LPS induction. Moreover, our findings help determine the sample size needed based on the immune population variability. In conclusion, the panels we have designed enable a comprehensive analysis of the murine immune system with a low blood volume requirement, enabling the measure of both absolute values and relative percentages effectively. This approach provides a robust platform for longitudinal studies in mice and can be used to uncover significant insights into immune responses.
Keywords: flow cytometry-methods; immune system; longitudinal studies; mice; peripheral blood.
Copyright © 2024 Barco-Tejada, López-Esteban, Mulero, Pion, Correa-Rocha, Desco and Cussó.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Cross-platform immunophenotyping of human peripheral blood mononuclear cells with four high-dimensional flow cytometry panels.Cytometry A. 2023 Jun;103(6):500-517. doi: 10.1002/cyto.a.24715. Epub 2023 Jan 13. Cytometry A. 2023. PMID: 36571245
-
Standardisation of flow cytometry for whole blood immunophenotyping of islet transplant and transplant clinical trial recipients.PLoS One. 2019 May 22;14(5):e0217163. doi: 10.1371/journal.pone.0217163. eCollection 2019. PLoS One. 2019. PMID: 31116766 Free PMC article.
-
One-Tube Multicolor Flow Cytometry Assay (OTMA) for Comprehensive Immunophenotyping of Peripheral Blood.Methods Mol Biol. 2019;1904:189-212. doi: 10.1007/978-1-4939-8958-4_8. Methods Mol Biol. 2019. PMID: 30539471
-
Flow cytometry immunophenotyping in integrated diagnostics of patients with newly diagnosed cytopenia: one tube 10-color 14-antibody screening panel and 3-tube extensive panel for detection of MDS-related features.Int J Lab Hematol. 2015 May;37 Suppl 1:133-43. doi: 10.1111/ijlh.12368. Int J Lab Hematol. 2015. PMID: 25976971 Review.
-
The role of flow cytometry in companion animal diagnostic medicine.Vet J. 2005 Nov;170(3):278-88. doi: 10.1016/j.tvjl.2004.06.010. Vet J. 2005. PMID: 16266842 Review.
References
-
- Fleisher T, Oliveira J. Flow cytometry. Clinical Immunology (Fifth Edition). (2019), 1239–51. doi: 10.1016/B978-0-7020-6896-6.00092-2 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

