Nifedipine Improves the Ketogenic Diet Effect on Insulin-Resistance-Induced Cognitive Dysfunction in Rats
- PMID: 39204160
- PMCID: PMC11359371
- DOI: 10.3390/ph17081054
Nifedipine Improves the Ketogenic Diet Effect on Insulin-Resistance-Induced Cognitive Dysfunction in Rats
Abstract
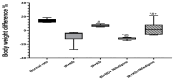
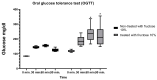
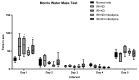
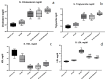
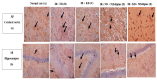
Insulin resistance, induced by high fructose consumption, affects cognitive function negatively. Nifedipine may be suggested for neurological disorders. This study aimed to assess the effect of nifedipine with either a normal diet (ND) or a ketogenic diet (KD) in cognitive dysfunction. Male Wistar rats received 10% fructose in drinking water for 8 weeks to induce insulin resistance. Rats received nifedipine (5.2 mg/kg/day; p.o.) later with ND or KD for an additional five weeks. One and two-way ANOVAs were used in analyzing the data. Reversion to the ND improved insulin resistance and lipid profile, besides brain-derived neurotrophic factor (BDNF), glycogen synthase kinase-3 beta (GSK3β), and insulin-degrading enzyme (IDE) levels. Rats fed KD alone and those that received nifedipine with KD did not show similar improvement in the previously mentioned parameters as the ND group. However, nifedipine-ND rats showed improvement in cognitive behavior and insulin resistance. Treatment with nifedipine-KD ameliorated GSK3β, amyloid β (Aβ), and tau protein levels. As the nifedipine-KD combination succeeded in diminishing the accumulated Aβ and tau protein, KD may be used for a while due to its side effects, then nifedipine treatment could be continued with an ND. This conclusion is based on the finding that this combination mitigated insulin resistance with the associated improved behavior.
Keywords: cognitive dysfunction; fructose; insulin resistance; ketogenic diet; nifedipine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Antiseizure Effects of Ketogenic Diet on Seizures Induced with Pentylenetetrazole, 4-Aminopyridine and Strychnine in Wistar Rats.Niger J Physiol Sci. 2017 Mar 6;31(2):115-119. Niger J Physiol Sci. 2017. PMID: 28262846
-
Effect of pioglitazone on altered expression of Aβ metabolism-associated molecules in the brain of fructose-drinking rats, a rodent model of insulin resistance.Eur J Pharmacol. 2011 Aug 16;664(1-3):14-9. doi: 10.1016/j.ejphar.2011.04.045. Epub 2011 May 3. Eur J Pharmacol. 2011. PMID: 21549699
-
The ketogenic diet prevents steatosis and insulin resistance by reducing lipogenesis, diacylglycerol accumulation and protein kinase C activity in male rat liver.J Physiol. 2022 Sep;600(18):4137-4151. doi: 10.1113/JP283552. Epub 2022 Sep 4. J Physiol. 2022. PMID: 35974660
-
Ketogenic diet alleviates β-cell dedifferentiation but aggravates hepatic lipid accumulation in db/db mice.Nutrition. 2024 Mar;119:112284. doi: 10.1016/j.nut.2023.112284. Epub 2023 Oct 31. Nutrition. 2024. PMID: 38118383
-
Ketogenic diet change cPLA2/clusterin and autophagy related gene expression and correlate with cognitive deficits and hippocampal MFs sprouting following neonatal seizures.Epilepsy Res. 2016 Feb;120:13-8. doi: 10.1016/j.eplepsyres.2015.11.021. Epub 2015 Dec 3. Epilepsy Res. 2016. PMID: 26709877
References
-
- Vargas E., Joy N.V., Sepulveda M.A.C. Biochemistry, Insulin Metabolic Effects. StatPearls Publishing; Treasure Island, FL, USA: 2023. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

