Chimeric Antigen Receptor T Cell Bearing Herpes Virus Entry Mediator Co-Stimulatory Signal Domain Exhibits Exhaustion-Resistant Properties
- PMID: 39201348
- PMCID: PMC11354286
- DOI: 10.3390/ijms25168662
Chimeric Antigen Receptor T Cell Bearing Herpes Virus Entry Mediator Co-Stimulatory Signal Domain Exhibits Exhaustion-Resistant Properties
Abstract
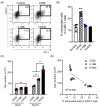
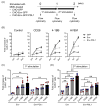
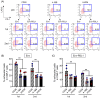
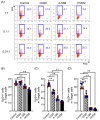
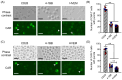
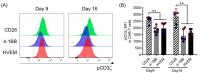
Improving chimeric antigen receptor (CAR)-T cell therapeutic outcomes and expanding its applicability to solid tumors requires further refinement of CAR-T cells. We previously reported that CAR-T cells bearing a herpes virus entry mediator (HVEM)-derived co-stimulatory signal domain (CSSD) (HVEM-CAR-T cells) exhibit superior functions and characteristics. Here, we conducted comparative analyses to evaluate the impact of different CSSDs on CAR-T cell exhaustion. The results indicated that HVEM-CAR-T cells had significantly lower frequencies of exhausted cells and exhibited the highest proliferation rates upon antigenic stimulation. Furthermore, proliferation inhibition by programmed cell death ligand 1 was stronger in CAR-T cells bearing CD28-derived CSSD (CD28-CAR-T cells) whereas it was weaker in HVEM-CAR-T. Additionally, HVEM-CAR-T cells maintained a low exhaustion level even after antigen-dependent proliferation and exhibited potent killing activities, suggesting that HVEM-CAR-T cells might be less prone to early exhaustion. Analysis of CAR localization on the cell surface revealed that CAR formed clusters in CD28-CAR-T cells whereas uniformly distributed in HVEM-CAR-T cells. Analysis of CD3ζ phosphorylation indicated that CAR-dependent tonic signals were strongly sustained in CD28-CAR-T cells whereas they were significantly weaker in HVEM-CAR-T cells. Collectively, these results suggest that the HVEM-derived CSSD is useful for generating CAR-T cells with exhaustion-resistant properties, which could be effective against solid tumors.
Keywords: T cell exhaustion; T cell-mediated immunotherapy; chimeric antigen receptor; herpes virus entry mediator; tonic signaling.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Chimeric Antigen Receptor T Cell Bearing Herpes Virus Entry Mediator Co-stimulatory Signal Domain Exhibits High Functional Potency.Mol Ther Oncolytics. 2019 Mar 23;14:27-37. doi: 10.1016/j.omto.2019.03.002. eCollection 2019 Sep 27. Mol Ther Oncolytics. 2019. PMID: 31011630 Free PMC article.
-
Herpes Virus Entry Mediator Costimulation Signaling Enhances CAR T-cell Efficacy Against Solid Tumors Through Metabolic Reprogramming.Cancer Immunol Res. 2023 Apr 3;11(4):515-529. doi: 10.1158/2326-6066.CIR-22-0531. Cancer Immunol Res. 2023. PMID: 36689620
-
4-1BB and optimized CD28 co-stimulation enhances function of human mono-specific and bi-specific third-generation CAR T cells.J Immunother Cancer. 2021 Oct;9(10):e003354. doi: 10.1136/jitc-2021-003354. J Immunother Cancer. 2021. PMID: 34706886 Free PMC article.
-
Chimeric Antigen Receptor T Cell Exhaustion during Treatment for Hematological Malignancies.Biomed Res Int. 2020 Oct 23;2020:8765028. doi: 10.1155/2020/8765028. eCollection 2020. Biomed Res Int. 2020. PMID: 33150182 Free PMC article. Review.
-
Engineering Cytoplasmic Signaling of CD28ζ CARs for Improved Therapeutic Functions.Front Immunol. 2020 Jun 19;11:1046. doi: 10.3389/fimmu.2020.01046. eCollection 2020. Front Immunol. 2020. PMID: 32636832 Free PMC article. Review.
References
-
- Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

