Xenografting Human Musculoskeletal Sarcomas in Mice, Chick Embryo, and Zebrafish: How to Boost Translational Research
- PMID: 39200384
- PMCID: PMC11352184
- DOI: 10.3390/biomedicines12081921
Xenografting Human Musculoskeletal Sarcomas in Mice, Chick Embryo, and Zebrafish: How to Boost Translational Research
Abstract
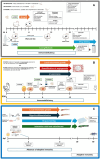
Musculoskeletal sarcomas pose major challenges to researchers and clinicians due to their rarity and heterogeneity. Xenografting human cells or tumor fragments in rodents is a mainstay for the generation of cancer models and for the preclinical trial of novel drugs. Lately, though, technical, intrinsic and ethical concerns together with stricter regulations have significantly curbed the employment of murine patient-derived xenografts (mPDX). In alternatives to murine PDXs, researchers have focused on embryonal systems such as chorioallantoic membrane (CAM) and zebrafish embryos. These systems are time- and cost-effective hosts for tumor fragments and near-patient cells. The CAM of the chick embryo represents a unique vascularized environment to host xenografts with high engraftment rates, allowing for ease of visualization and molecular detection of metastatic cells. Thanks to the transparency of the larvae, zebrafish allow for the tracking of tumor development and metastatization, enabling high-throughput drug screening. This review will focus on xenograft models of musculoskeletal sarcomas to highlight the intrinsic and technically distinctive features of the different hosts, and how they can be exploited to elucidate biological mechanisms beneath the different phases of the tumor's natural history and in drug development. Ultimately, the review suggests the combination of different models as an advantageous approach to boost basic and translational research.
Keywords: cell-derived xenografts (CDX); chorioallantoic membrane (CAM); murine models; musculoskeletal sarcomas; patient-derived xenografts (PDX); zebrafish.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
The chick chorioallantoic membrane (CAM) as a versatile patient-derived xenograft (PDX) platform for precision medicine and preclinical research.Am J Cancer Res. 2018 Aug 1;8(8):1642-1660. eCollection 2018. Am J Cancer Res. 2018. PMID: 30210932 Free PMC article. Review.
-
The chick embryo chorioallantoic membrane patient-derived xenograft (PDX) model.Pathol Res Pract. 2023 Mar;243:154367. doi: 10.1016/j.prp.2023.154367. Epub 2023 Feb 10. Pathol Res Pract. 2023. PMID: 36774760 Review.
-
Applications of the Chick Chorioallantoic Membrane as an Alternative Model for Cancer Studies.Cells Tissues Organs. 2022;211(2):222-237. doi: 10.1159/000513039. Epub 2021 Mar 29. Cells Tissues Organs. 2022. PMID: 33780951 Free PMC article. Review.
-
Chick Chorioallantoic Membrane as a Patient-Derived Xenograft Model for Uveal Melanoma: Imaging Modalities for Growth and Vascular Evaluation.Cancers (Basel). 2023 Feb 24;15(5):1436. doi: 10.3390/cancers15051436. Cancers (Basel). 2023. PMID: 36900228 Free PMC article.
-
Patient-Derived Xenograft Models for Translational Prostate Cancer Research and Drug Development.Methods Mol Biol. 2024;2806:153-185. doi: 10.1007/978-1-0716-3858-3_12. Methods Mol Biol. 2024. PMID: 38676802
References
-
- Stacchiotti S., Frezza A.M., Blay J., Baldini E.H., Bonvalot S., Bovée J.V.M.G., Callegaro D., Casali P.G., Chiang R.C., Demetri G.D., et al. Ultra-rare Sarcomas: A Consensus Paper from the Connective Tissue Oncology Society Community of Experts on the Incidence Threshold and the List of Entities. Cancer. 2021;127:2934–2942. doi: 10.1002/cncr.33618. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

