Bovine Placentome-Derived Extracellular Matrix: A Sustainable 3D Scaffold for Cultivated Meat
- PMID: 39199811
- PMCID: PMC11352162
- DOI: 10.3390/bioengineering11080854
Bovine Placentome-Derived Extracellular Matrix: A Sustainable 3D Scaffold for Cultivated Meat
Abstract
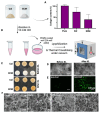
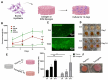
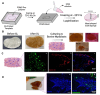
Cultivated meat, an advancement in cellular agriculture, holds promise in addressing environmental, ethical, and health challenges associated with traditional meat production. Utilizing tissue engineering principles, cultivated meat production employs biomaterials and technologies to create cell-based structures by introducing cells into a biocompatible scaffold, mimicking tissue organization. Among the cell sources used for producing muscle-like tissue for cultivated meats, primary adult stem cells like muscle satellite cells exhibit robust capabilities for proliferation and differentiation into myocytes, presenting a promising avenue for cultivated meat production. Evolutionarily optimized for growth in a 3D microenvironment, these cells benefit from the biochemical and biophysical cues provided by the extracellular matrix (ECM), regulating cell organization, interactions, and behavior. While plant protein-based scaffolds have been explored for their utilization for cultivated meat, they lack the biological cues for animal cells unless functionalized. Conversely, a decellularized bovine placental tissue ECM, processed from discarded birth tissue, achieves the biological functionalities of animal tissue ECM without harming animals. In this study, collagen and total ECM were prepared from decellularized bovine placental tissues. The collagen content was determined to be approximately 70% and 40% in isolated collagen and ECM, respectively. The resulting porous scaffolds, crosslinked through a dehydrothermal (DHT) crosslinking method without chemical crosslinking agents, supported the growth of bovine myoblasts. ECM scaffolds exhibited superior compatibility and stability compared to collagen scaffolds. In an attempt to make cultivate meat constructs, bovine myoblasts were cultured in steak-shaped ECM scaffolds for about 50 days. The resulting construct not only resembled muscle tissues but also displayed high cellularity with indications of myogenic differentiation. Furthermore, the meat constructs were cookable and able to sustain the grilling/frying. Our study is the first to utilize a unique bovine placentome-derived ECM scaffold to create a muscle tissue-like meat construct, demonstrating a promising and sustainable option for cultivated meat production.
Keywords: collagen; cultivated meat; dehydrothermal crosslinking (DHT); extracellular matrix; scaffolds; tissue engineering.
Conflict of interest statement
Author Mohit Bhatia was employed by the company Atelier Meats. This study was partially supported by Atelier Meats, Corporation. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures


Similar articles
-
Bovine Fibroblast-Derived Extracellular Matrix Promotes the Growth and Preserves the Stemness of Bovine Stromal Cells during In Vitro Expansion.J Funct Biomater. 2023 Apr 13;14(4):218. doi: 10.3390/jfb14040218. J Funct Biomater. 2023. PMID: 37103308 Free PMC article.
-
3D porous scaffolds from wheat glutenin for cultured meat applications.Biomaterials. 2022 Jun;285:121543. doi: 10.1016/j.biomaterials.2022.121543. Epub 2022 Apr 27. Biomaterials. 2022. PMID: 35533444
-
3D-printable plant protein-enriched scaffolds for cultivated meat development.Biomaterials. 2022 May;284:121487. doi: 10.1016/j.biomaterials.2022.121487. Epub 2022 Mar 24. Biomaterials. 2022. PMID: 35421802
-
Extracellular Matrix and the Production of Cultured Meat.Foods. 2021 Dec 15;10(12):3116. doi: 10.3390/foods10123116. Foods. 2021. PMID: 34945667 Free PMC article. Review.
-
Scaffolding fundamentals and recent advances in sustainable scaffolding techniques for cultured meat development.Food Res Int. 2024 Aug;189:114549. doi: 10.1016/j.foodres.2024.114549. Epub 2024 May 26. Food Res Int. 2024. PMID: 38876607 Review.
References
-
- Rojas-Downing M.M., Nejadhashemi A.P., Harrigan T., Woznicki S.A. Climate Change and Livestock: Impacts, Adaptation, and Mitigation. Clim. Risk Manag. 2017;16:145–163. doi: 10.1016/j.crm.2017.02.001. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

