Synergistic Strategies for Castration-Resistant Prostate Cancer: Targeting AR-V7, Exploring Natural Compounds, and Optimizing FDA-Approved Therapies
- PMID: 39199550
- PMCID: PMC11352813
- DOI: 10.3390/cancers16162777
Synergistic Strategies for Castration-Resistant Prostate Cancer: Targeting AR-V7, Exploring Natural Compounds, and Optimizing FDA-Approved Therapies
Abstract
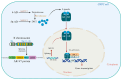
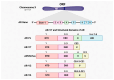
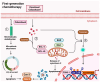
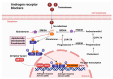
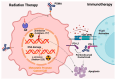
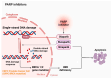
Castration-resistant prostate cancer (CRPC) remains a significant therapeutic challenge due to its resistance to standard androgen deprivation therapy (ADT). The emergence of androgen receptor splice variant 7 (AR-V7) has been implicated in CRPC progression, contributing to treatment resistance. Current treatments, including first-generation chemotherapy, androgen receptor blockers, radiation therapy, immune therapy, and PARP inhibitors, often come with substantial side effects and limited efficacy. Natural compounds, particularly those derived from herbal medicine, have garnered increasing interest as adjunctive therapeutic agents against CRPC. This review explores the role of AR-V7 in CRPC and highlights the promising benefits of natural compounds as complementary treatments to conventional drugs in reducing CRPC and overcoming therapeutic resistance. We delve into the mechanisms of action underlying the anti-CRPC effects of natural compounds, showcasing their potential to enhance therapeutic outcomes while mitigating the side effects associated with conventional therapies. The exploration of natural compounds offers promising avenues for developing novel treatment strategies that enhance therapeutic outcomes and reduce the adverse effects of conventional CRPC therapies. These compounds provide a safer, more effective approach to managing CRPC, representing a significant advancement in improving patient care.
Keywords: androgen receptor blockers; androgen receptor splice variant 7; castration-resistant prostate cancer; herbal medicine; natural compounds.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Analytical Validation and Clinical Qualification of a New Immunohistochemical Assay for Androgen Receptor Splice Variant-7 Protein Expression in Metastatic Castration-resistant Prostate Cancer.Eur Urol. 2016 Oct;70(4):599-608. doi: 10.1016/j.eururo.2016.03.049. Epub 2016 Apr 23. Eur Urol. 2016. PMID: 27117751 Free PMC article.
-
Prognostic Value of Androgen Receptor Splice Variant 7 in the Treatment of Castration-resistant Prostate Cancer with Next generation Androgen Receptor Signal Inhibition: A Systematic Review and Meta-analysis.Eur Urol Focus. 2018 Jul;4(4):529-539. doi: 10.1016/j.euf.2017.01.004. Epub 2017 Jan 23. Eur Urol Focus. 2018. PMID: 28753843
-
Transcript Levels of Androgen Receptor Variant 7 and Ubiquitin-Conjugating Enzyme 2C in Hormone Sensitive Prostate Cancer and Castration-Resistant Prostate Cancer.Prostate. 2017 Jan;77(1):60-71. doi: 10.1002/pros.23248. Epub 2016 Aug 22. Prostate. 2017. PMID: 27550197
-
The Crucial Role of AR-V7 in Enzalutamide-Resistance of Castration-Resistant Prostate Cancer.Cancers (Basel). 2022 Oct 5;14(19):4877. doi: 10.3390/cancers14194877. Cancers (Basel). 2022. PMID: 36230800 Free PMC article. Review.
-
[ARV-7: A biomarker for the treatment of metastatic castration-resistant prostate cancer].Zhonghua Nan Ke Xue. 2019 Feb;25(2):172-176. Zhonghua Nan Ke Xue. 2019. PMID: 32216207 Review. Chinese.
References
-
- Wang Z., Yan X., Tang P., Tang T., Wang Y., Peng S., Wang S., Lan W., Wang L., Zhang Y. Genetic profiling of hormone-sensitive and castration-resistant prostate cancers and identification of genetic mutations prone to castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2023;26:180–187. doi: 10.1038/s41391-022-00618-2. - DOI - PubMed
-
- Karaca M. Regulation of Androgen Receptor Function by Tyrosine Phosphorylation. 2010. [(accessed on 18 March 2013)]. Available online: https://cdr.lib.unc.edu/concern/dissertations/dr26xz50m.
Publication types
Grants and funding
- NRF-2020R1I1A2066868/Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education
- 2020R1A5A2019413/National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT)
- RS-2020-KH087790/Ministry of Health & Welfare, Republic of Korea
- RS-2024-00350362/national Research Foundation of korea (NRF) grant funded by the korea Government (MSIT)
LinkOut - more resources
Full Text Sources
Research Materials

