Expression of Foxtail Millet bZIP Transcription Factor SibZIP67 Enhances Drought Tolerance in Arabidopsis
- PMID: 39199345
- PMCID: PMC11352937
- DOI: 10.3390/biom14080958
Expression of Foxtail Millet bZIP Transcription Factor SibZIP67 Enhances Drought Tolerance in Arabidopsis
Abstract
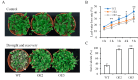
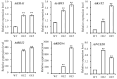
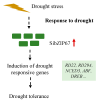
Foxtail millet is a drought-tolerant cereal and forage crop. The basic leucine zipper (bZIP) gene family plays important roles in regulating plant development and responding to stresses. However, the roles of bZIP genes in foxtail millet remain largely uninvestigated. In this study, 92 members of the bZIP transcription factors were identified in foxtail millet and clustered into ten clades. The expression levels of four SibZIP genes (SibZIP11, SibZIP12, SibZIP41, and SibZIP67) were significantly induced after PEG treatment, and SibZIP67 was chosen for further analysis. The studies showed that ectopic overexpression of SibZIP67 in Arabidopsis enhanced the plant drought tolerance. Detached leaves of SibZIP67 overexpressing plants had lower leaf water loss rates than those of wild-type plants. SibZIP67 overexpressing plants improved survival rates under drought conditions compared to wild-type plants. Additionally, overexpressing SibZIP67 in plants displayed reduced malondialdehyde (MDA) levels and enhanced activities of antioxidant enzymes, including catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD) under drought stress. Furthermore, the drought-related genes, such as AtRD29A, AtRD22, AtNCED3, AtABF3, AtABI1, and AtABI5, were found to be regulated in SibZIP67 transgenic plants than in wild-type Arabidopsis under drought conditions. These data suggested that SibZIP67 conferred drought tolerance in transgenic Arabidopsis by regulating antioxidant enzyme activities and the expression of stress-related genes. The study reveals that SibZIP67 plays a beneficial role in drought response in plants, offering a valuable genetic resource for agricultural improvement in arid environments.
Keywords: Setaria italica; bZIP; drought; transcription factor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
[MYB-like transcription factor SiMYB42 from foxtail millet (Setaria italica L.) enhances Arabidopsis tolerance to low-nitrogen stress].Yi Chuan. 2018 Apr 20;40(4):327-338. doi: 10.16288/j.yczz.17-315. Yi Chuan. 2018. PMID: 29704378 Chinese.
-
Overexpression of the autophagy-related gene SiATG8a from foxtail millet (Setaria italica L.) confers tolerance to both nitrogen starvation and drought stress in Arabidopsis.Biochem Biophys Res Commun. 2015 Dec 25;468(4):800-6. doi: 10.1016/j.bbrc.2015.11.035. Epub 2015 Nov 11. Biochem Biophys Res Commun. 2015. PMID: 26577407
-
Genome-wide identification of the MED25 BINDING RING-H2 PROTEIN gene family in foxtail millet (Setaria italica L.) and the role of SiMBR2 in resistance to abiotic stress in Arabidopsis.Planta. 2024 Jun 7;260(1):22. doi: 10.1007/s00425-024-04455-6. Planta. 2024. PMID: 38847958
-
An ABA-responsive DRE-binding protein gene from Setaria italica, SiARDP, the target gene of SiAREB, plays a critical role under drought stress.J Exp Bot. 2014 Oct;65(18):5415-27. doi: 10.1093/jxb/eru302. Epub 2014 Jul 28. J Exp Bot. 2014. PMID: 25071221 Free PMC article.
-
Effect of drought acclimation on antioxidant system and polyphenolic content of Foxtail Millet (Setaria italica L.).Physiol Mol Biol Plants. 2023 Oct;29(10):1577-1589. doi: 10.1007/s12298-023-01366-w. Epub 2023 Oct 5. Physiol Mol Biol Plants. 2023. PMID: 38076760 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

