Actin Cytoskeleton and Integrin Components Are Interdependent for Slit Diaphragm Maintenance in Drosophila Nephrocytes
- PMID: 39195240
- PMCID: PMC11352372
- DOI: 10.3390/cells13161350
Actin Cytoskeleton and Integrin Components Are Interdependent for Slit Diaphragm Maintenance in Drosophila Nephrocytes
Abstract
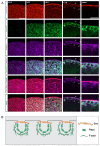
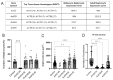
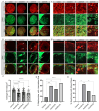
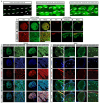
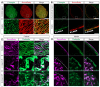
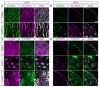
In nephrotic syndrome, the podocyte filtration structures are damaged in a process called foot process effacement. This is mediated by the actin cytoskeleton; however, which actins are involved and how they interact with other filtration components, like the basement membrane, remains poorly understood. Here, we used the well-established Drosophila pericardial nephrocyte-the equivalent of podocytes in flies-knockdown models (RNAi) to study the interplay of the actin cytoskeleton (Act5C, Act57B, Act42A, and Act87E), alpha- and beta-integrin (basement membrane), and the slit diaphragm (Sns and Pyd). Knockdown of an actin gene led to variations of formation of actin stress fibers, the internalization of Sns, and a disrupted slit diaphragm cortical pattern. Notably, deficiency of Act5C, which resulted in complete absence of nephrocytes, could be partially mitigated by overexpressing Act42A or Act87E, suggesting at least partial functional redundancy. Integrin localized near the actin cytoskeleton as well as slit diaphragm components, but when the nephrocyte cytoskeleton or slit diaphragm was disrupted, this switched to colocalization, both at the surface and internalized in aggregates. Altogether, the data show that the interdependence of the slit diaphragm, actin cytoskeleton, and integrins is key to the structure and function of the Drosophila nephrocyte.
Keywords: Drosophila; actin cytoskeleton; integrin; lacunar channel; nephrocyte; piezo; slit diaphragm.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm.Nature. 2009 Jan 15;457(7227):322-6. doi: 10.1038/nature07526. Epub 2008 Oct 29. Nature. 2009. PMID: 18971929 Free PMC article.
-
Nephrin Signaling Results in Integrin β1 Activation.J Am Soc Nephrol. 2019 Jun;30(6):1006-1019. doi: 10.1681/ASN.2018040362. Epub 2019 May 16. J Am Soc Nephrol. 2019. PMID: 31097607 Free PMC article.
-
Networks that link cytoskeletal regulators and diaphragm proteins underpin filtration function in Drosophila nephrocytes.Exp Cell Res. 2018 Mar 15;364(2):234-242. doi: 10.1016/j.yexcr.2018.02.015. Epub 2018 Feb 16. Exp Cell Res. 2018. PMID: 29458174 Free PMC article.
-
Regulation of the Actin Cytoskeleton in Podocytes.Cells. 2020 Jul 16;9(7):1700. doi: 10.3390/cells9071700. Cells. 2020. PMID: 32708597 Free PMC article. Review.
-
Actin dynamics at focal adhesions: a common endpoint and putative therapeutic target for proteinuric kidney diseases.Kidney Int. 2018 Jun;93(6):1298-1307. doi: 10.1016/j.kint.2017.12.028. Epub 2018 Apr 17. Kidney Int. 2018. PMID: 29678354 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

