Molecular screening of transitional B cells as a prognostic marker of improved graft outcome and reduced rejection risk in kidney transplant
- PMID: 39192987
- PMCID: PMC11348389
- DOI: 10.3389/fimmu.2024.1433832
Molecular screening of transitional B cells as a prognostic marker of improved graft outcome and reduced rejection risk in kidney transplant
Abstract
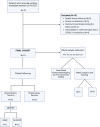
Introduction: Understanding immune cell dynamics in kidney transplantation may provide insight into the mechanisms of rejection and improve patient management. B cells have gained interest with a special relevance of the "regulatory" subsets and their graft outcome prognostic value. In this study, we aimed to prove that the direct immunophenotyping and target gene expression analysis of kidney transplant patients' fresh whole blood will help to identify graft rejection risk and assist in the monitoring of kidney transplanted patients.
Methods: We employed flow cytometry and qPCR techniques to characterize B and T cell subsets within fresh whole blood samples, with particular emphasis on transitional B cells (TrB) identified as CD19+CD24hiCD38hi. TrB are a relevant population in the context of kidney transplantation and are closely associated with regulatory B cells (Bregs) in humans. Patients were monitored, tracking pertinent clinical parameters and kidney-related events, including alterations in graft function and episodes of biopsy proven rejection.
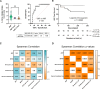
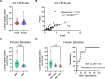
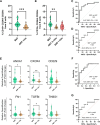
Results: Higher percentages of TrB cells at 3 months after transplantation were positively associated with better graft outcomes and lower biopsy-proven acute rejection risk. Furthermore, a novel panel of B cell regulatory associated genes was validated at 3 months post-transplantation by qPCR analysis of peripheral blood mononuclear cell (PBMC) mRNA, showing high predictive power of graft events and prognostic value.
Discussion: These findings suggest that monitoring TrB may provide interesting patient management information, improve transplant outcomes, and allow for personalized drug regimens to minimize clinical complications.
Keywords: Breg; acute graft rejection; flow cytometry; immunophenotyping; transitional B cells.
Copyright © 2024 Perezpayá, Garcia, Clos-Sansalvador, Sanroque-Muñoz, Font-Morón, Rodríguez-Martínez, Vila-Santandreu, Bover, Borràs, Cañas and Franquesa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author MF declares that they were an editorial board member of Frontiers, at the time of submission. Thishad no impact on the peer review process and the final decision.
Figures


Similar articles
-
Transitional immature regulatory B cells and regulatory cytokines can discriminate chronic antibody-mediated rejection from stable graft function.Int Immunopharmacol. 2020 Sep;86:106750. doi: 10.1016/j.intimp.2020.106750. Epub 2020 Jul 8. Int Immunopharmacol. 2020. PMID: 32652501
-
The balance between memory and regulatory cell populations in kidney transplant recipients with operational tolerance.Clin Exp Immunol. 2024 May 16;216(3):318-330. doi: 10.1093/cei/uxae018. Clin Exp Immunol. 2024. PMID: 38393856
-
Immunosuppression Has Long-Lasting Effects on Circulating Follicular Regulatory T Cells in Kidney Transplant Recipients.Front Immunol. 2020 Aug 28;11:1972. doi: 10.3389/fimmu.2020.01972. eCollection 2020. Front Immunol. 2020. PMID: 32983131 Free PMC article.
-
Urinary Cell mRNA Profiles Predictive of Human Kidney Allograft Status.Clin J Am Soc Nephrol. 2021 Oct;16(10):1565-1577. doi: 10.2215/CJN.14010820. Epub 2021 Apr 27. Clin J Am Soc Nephrol. 2021. PMID: 33906907 Free PMC article. Review.
-
The regulatory roles of B cell subsets in transplantation.Expert Rev Clin Immunol. 2018 Feb;14(2):115-125. doi: 10.1080/1744666X.2018.1426461. Epub 2018 Jan 23. Expert Rev Clin Immunol. 2018. PMID: 29338551 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

