The TGFβ type I receptor kinase inhibitor vactosertib in combination with pomalidomide in relapsed/refractory multiple myeloma: a phase 1b trial
- PMID: 39191755
- PMCID: PMC11350185
- DOI: 10.1038/s41467-024-51442-2
The TGFβ type I receptor kinase inhibitor vactosertib in combination with pomalidomide in relapsed/refractory multiple myeloma: a phase 1b trial
Abstract
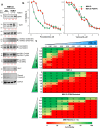
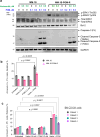
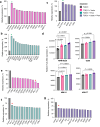
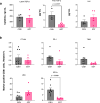
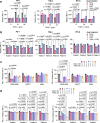
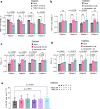
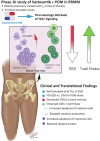
Functional blockade of the transforming growth factor-beta (TGFβ) signalling pathway improves the efficacy of cytotoxic and immunotherapies. Here, we conducted a phase 1b study (ClinicalTrials.gov., NCT03143985) to determine the primary endpoints of safety, tolerability, and maximal tolerated dose (200 mg twice daily) for the orally-available TGFβ type I receptor kinase inhibitor vactosertib in combination with pomalidomide in relapsed and/or refractory multiple myeloma (RRMM) patients who had received ≥2 lines of chemoimmunotherapy. Secondary endpoints demonstrated sustained clinical responses, favorable pharmacokinetic parameters and a 6-month progression-free survival of 82%. Vactosertib combined with pomalidomide was well-tolerated at all dose levels and displayed a manageable adverse event profile. Exploratory analysis indicated that vactosertib co-treatment with pomalidomide also reduced TGFβ levels in patient bone marrow as well as the level of CD8+ T-cells that expressed the immunoinhibitory marker PD-1. In vitro experiments indicated that vactosertib+pomalidomide co-treatment decreased the viability of MM cell lines and patient tumor cells, and increased CD8+ T-cell cytotoxic activity. Vactosertib is a safe therapeutic that demonstrates tumor-intrinsic activity and can overcome immunosuppressive challenges within the tumor microenvironment to reinvigorate T-cell fitness. Vactosertib offers promise to improve immunotherapeutic responses in heavily-pretreated MM patients refractory to conventional agents.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Update of
-
Vactosertib, a novel TGF-β1 type I receptor kinase inhibitor, improves T-cell fitness: a single-arm, phase 1b trial in relapsed/refractory multiple myeloma.Res Sq [Preprint]. 2023 Jul 17:rs.3.rs-3112163. doi: 10.21203/rs.3.rs-3112163/v1. Res Sq. 2023. Update in: Nat Commun. 2024 Aug 27;15(1):7388. doi: 10.1038/s41467-024-51442-2 PMID: 37503043 Free PMC article. Updated. Preprint.
Similar articles
-
Vactosertib, a novel TGF-β1 type I receptor kinase inhibitor, improves T-cell fitness: a single-arm, phase 1b trial in relapsed/refractory multiple myeloma.Res Sq [Preprint]. 2023 Jul 17:rs.3.rs-3112163. doi: 10.21203/rs.3.rs-3112163/v1. Res Sq. 2023. Update in: Nat Commun. 2024 Aug 27;15(1):7388. doi: 10.1038/s41467-024-51442-2 PMID: 37503043 Free PMC article. Updated. Preprint.
-
Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study.Lancet. 2019 Dec 7;394(10214):2096-2107. doi: 10.1016/S0140-6736(19)32556-5. Epub 2019 Nov 14. Lancet. 2019. PMID: 31735560 Clinical Trial.
-
Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial.Lancet Oncol. 2019 Jun;20(6):781-794. doi: 10.1016/S1470-2045(19)30152-4. Epub 2019 May 13. Lancet Oncol. 2019. PMID: 31097405 Clinical Trial.
-
Pomalidomide: A Review in Relapsed and Refractory Multiple Myeloma.Drugs. 2017 Nov;77(17):1897-1908. doi: 10.1007/s40265-017-0833-y. Drugs. 2017. PMID: 29110190 Review.
-
Pomalidomide: a review of its use in patients with recurrent multiple myeloma.Drugs. 2014 Apr;74(5):549-62. doi: 10.1007/s40265-014-0196-6. Drugs. 2014. PMID: 24590685 Review.
References
-
- Mateos, M. V., Nooka, A. K. & Larson, S. M. Moving toward a cure for myeloma. Am. Soc. Clin. Oncol. Educ. Book42, 1–12 (2022). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

