Endoplasmic reticulum stress in diseases
- PMID: 39188936
- PMCID: PMC11345536
- DOI: 10.1002/mco2.701
Endoplasmic reticulum stress in diseases
Abstract
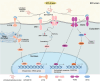
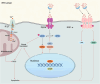
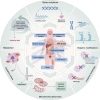
The endoplasmic reticulum (ER) is a key organelle in eukaryotic cells, responsible for a wide range of vital functions, including the modification, folding, and trafficking of proteins, as well as the biosynthesis of lipids and the maintenance of intracellular calcium homeostasis. A variety of factors can disrupt the function of the ER, leading to the aggregation of unfolded and misfolded proteins within its confines and the induction of ER stress. A conserved cascade of signaling events known as the unfolded protein response (UPR) has evolved to relieve the burden within the ER and restore ER homeostasis. However, these processes can culminate in cell death while ER stress is sustained over an extended period and at elevated levels. This review summarizes the potential role of ER stress and the UPR in determining cell fate and function in various diseases, including cardiovascular diseases, neurodegenerative diseases, metabolic diseases, autoimmune diseases, fibrotic diseases, viral infections, and cancer. It also puts forward that the manipulation of this intricate signaling pathway may represent a novel target for drug discovery and innovative therapeutic strategies in the context of human diseases.
Keywords: diseases; endoplasmic reticulum stress (ER stress); therapeutic strategies; unfolded protein response (UPR).
© 2024 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Protein-rich foods, sea foods, and gut microbiota amplify immune responses in chronic diseases and cancers - Targeting PERK as a novel therapeutic strategy for chronic inflammatory diseases, neurodegenerative disorders, and cancer.Pharmacol Ther. 2024 Mar;255:108604. doi: 10.1016/j.pharmthera.2024.108604. Epub 2024 Feb 13. Pharmacol Ther. 2024. PMID: 38360205 Review.
-
The endoplasmic reticulum: Homeostasis and crosstalk in retinal health and disease.Prog Retin Eye Res. 2024 Jan;98:101231. doi: 10.1016/j.preteyeres.2023.101231. Epub 2023 Dec 12. Prog Retin Eye Res. 2024. PMID: 38092262 Free PMC article. Review.
-
Endoplasmic reticulum stress and the unfolded protein responses in retinal degeneration.Exp Eye Res. 2014 Aug;125:30-40. doi: 10.1016/j.exer.2014.04.015. Epub 2014 May 2. Exp Eye Res. 2014. PMID: 24792589 Free PMC article. Review.
-
Hidden Agenda - The Involvement of Endoplasmic Reticulum Stress and Unfolded Protein Response in Inflammation-Induced Muscle Wasting.Front Immunol. 2022 May 9;13:878755. doi: 10.3389/fimmu.2022.878755. eCollection 2022. Front Immunol. 2022. PMID: 35615361 Free PMC article. Review.
-
Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases.Diabetes Obes Metab. 2010 Oct;12 Suppl 2:108-15. doi: 10.1111/j.1463-1326.2010.01282.x. Diabetes Obes Metab. 2010. PMID: 21029307 Review.
References
-
- Porter KR, Kallman FL. Significance of cell particulates as seen by electron microscopy. Ann NY Acad Sci. 1952;54(6):882‐891. - PubMed
-
- MacLennan DH, Rice WJ, Green NM. The mechanism of Ca2+ transport by sarco(endo)plasmic reticulum Ca2+‐ATPases. J Biol Chem. 1997;272(46):28815‐28818. - PubMed
-
- Wang CC. Protein disulfide isomerase assists protein folding as both an isomerase and a chaperone. Ann NY Acad Sci. 1998;864:9‐13. - PubMed
-
- Harootunian AT, Kao JP, Paranjape S, Tsien RY. Generation of calcium oscillations in fibroblasts by positive feedback between calcium and IP3. Science. 1991;251(4989):75‐78. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
