Monitoring the VDPV2 outbreak in Egypt during 2020-2021 highlights the crucial role of environmental surveillance and boosting immunization in combating Poliovirus
- PMID: 39187787
- PMCID: PMC11348703
- DOI: 10.1186/s12879-024-09731-0
Monitoring the VDPV2 outbreak in Egypt during 2020-2021 highlights the crucial role of environmental surveillance and boosting immunization in combating Poliovirus
Abstract
Background: Poliovirus is a highly infectious enterovirus (EV) that primarily affects children and can lead to lifelong paralysis or even death. Vaccine-derived polioviruses (VDPVs) are a great threat since they are derived from the attenuated virus in the Oral Poliovirus Vaccine (OPV) and can mutate to a more virulent form. The purpose of this study was to identify VDPV serotype 2 through the year 2020-2021 via surveillance of sewage samples collected from different localities and governorates in Egypt and stool specimens from Acute Flaccid Paralysis (AFP) cases. Both were collected through the national poliovirus surveillance system and according to the guidelines recommended by the WHO.
Methods: A total of 1266 sewage samples and 3241 stool samples from January 2020 to December 2021 were investigated in the lab according to World Health Organization (WHO) protocol for the presence of Polioviruses by cell culture, molecular identification of positive isolates on L20B cell line was carried out using real-time polymerase chain reactions (RT-PCR). Any positive isolates for Poliovirus type 2 and isolates suspected of Vaccine Derived Poliovirus Type 1 and type 3 screened by (VDPV1) or Vaccine Poliovirus Type 3 (VDPV3) assay in RT-PCR were referred for VP1 genetic sequencing.
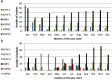
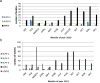
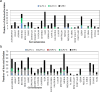
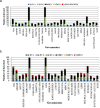
Results: The outbreak was caused by circulating VDPV2 (cVDPV2) strains started in January 2021. By the end of February 2021, a total of 11 cVDPV2s were detected in sewage samples from six governorates confirming the outbreak situation. One additional cVDPV2 was detected later in the sewage sample from Qena (June 2021). The first and only re-emergence of VDPV2 in stool samples during the outbreak was in contact with Luxor in June 2021. By November 2021, a total of 80 VDPVs were detected. The Egyptian Ministry of Health and Population (MOHP), in collaboration with the WHO, responded quickly by launching two massive vaccination campaigns targeting children under the age of five. Additionally, surveillance systems were strengthened to detect new cases and prevent further spread of the virus.
Conclusion: The continued threat of poliovirus and VDPVs requires ongoing efforts to prevent their emergence and spread. Strategies such as improving immunization coverage, using genetically stable vaccines, and establishing surveillance systems are critical to achieving global eradication of poliovirus and efficient monitoring of VDPVs outbreaks.
Keywords: Environmental surveillance; Oral poliovirus vaccine; Sewage; Stool; Vaccine-derived polioviruses (VDPVs); World Health Organization.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Type 2 Poliovirus Detection after Global Withdrawal of Trivalent Oral Vaccine.N Engl J Med. 2018 Aug 30;379(9):834-845. doi: 10.1056/NEJMoa1716677. N Engl J Med. 2018. PMID: 30157398 Free PMC article.
-
Environmental surveillance of a circulating vaccine-derived poliovirus type 2 outbreak in Israel between 2022 and 2023: a genomic epidemiology study.Lancet Microbe. 2024 Oct;5(10):100893. doi: 10.1016/S2666-5247(24)00116-2. Epub 2024 Sep 13. Lancet Microbe. 2024. PMID: 39284332
-
Update on Vaccine-Derived Polioviruses - Worldwide, January 2014-March 2015.MMWR Morb Mortal Wkly Rep. 2015 Jun 19;64(23):640-6. MMWR Morb Mortal Wkly Rep. 2015. PMID: 26086635 Free PMC article.
-
Circulating vaccine-derived polioviruses: current state of knowledge.Bull World Health Organ. 2004 Jan;82(1):16-23. Epub 2004 Feb 26. Bull World Health Organ. 2004. PMID: 15106296 Free PMC article. Review.
-
Responding to a cVDPV1 outbreak in Ukraine: Implications, challenges and opportunities.Vaccine. 2017 Aug 24;35(36):4769-4776. doi: 10.1016/j.vaccine.2017.04.036. Epub 2017 May 19. Vaccine. 2017. PMID: 28528761 Free PMC article. Review.
References
-
- Li XM, Zhang ZJ, Wang HH, Liu F, Zhang LW, Chu P, et al. Immunogenicity and safety of a booster dose of inactivated polio vaccine. Zhonghua Yu Fang Yi Xue Za Zhi. 2013;47(10):905–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

