This is a preprint.
Microglia are Required for Developmental Specification of AgRP Innervation in the Hypothalamus of Offspring Exposed to Maternal High Fat Diet During Lactation
- PMID: 39185162
- PMCID: PMC11343114
- DOI: 10.1101/2024.08.12.607566
Microglia are Required for Developmental Specification of AgRP Innervation in the Hypothalamus of Offspring Exposed to Maternal High Fat Diet During Lactation
Abstract
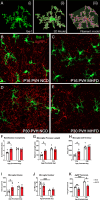
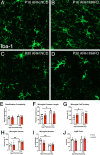
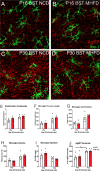
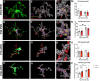
Nutritional fluctuations that occur early in life dictate metabolic adaptations that will affect susceptibility to weight gain and obesity later in life. The postnatal period in mice represents a time of dynamic changes in hypothalamic development and maternal consumption of a high fat diet during the lactation period (MHFD) changes the composition of milk and leads to enhanced susceptibility to obesity in offspring. Agouti-related peptide (AgRP) neurons in the arcuate nucleus of the hypothalamus (ARH) react to changes in multiple metabolic signals and distribute neuroendocrine information to other brain regions, such as the paraventricular hypothalamic nucleus (PVH), which is known to integrate a variety of signals that regulate body weight. Development of neural projections from AgRP neurons to the PVH occurs during the lactation period and these projections are reduced in MHFD offspring, but underlying developmental mechanisms remain largely unknown. Microglia are the resident immune cells of the central nervous system and are involved in refinement of neural connections and modulation of synaptic transmission. Because high fat diet exposure causes activation of microglia in adults, a similar activation may occur in offspring exposed to MHFD and play a role in sculpting hypothalamic feeding circuitry. Genetically targeted axonal labeling and immunohistochemistry were used to visualize AgRP axons and microglia in postnatal mice derived from MHFD dams and morphological changes quantified. The results demonstrate regionally localized changes to microglial morphology in the PVH of MHFD offspring that suggest enhanced surveillance activity and are temporally restricted to the period when AgRP neurons innervate the PVH. In addition, axon labeling experiments confirm a significant decrease in AgRP innervation of the PVH in MHFD offspring and provide direct evidence of synaptic pruning of AgRP inputs to the PVH. Microglial depletion with the Colony-stimulating factor 1 receptor inhibitor PLX5622 determined that the decrease in AgRP innervation observed in MHFD offspring is dependent on microglia, and that microglia are required for weight gain that emerges as early as weaning in offspring of MHFD dams. However, these changes do not appear to be dependent on the degree of microglial mediated synaptic pruning. Together, these findings suggest that microglia are activated by exposure to MHFD and interact directly with AgRP axons during development to permanently alter their density, with implications for developmental programming of metabolic phenotype.
Keywords: Agouti-related peptide; Developmental Biology; Hypothalamus; Major category; Microglia; Minor category; Neural Development; Neuroscience; Paraventricular hypothalamic nucleus.
Conflict of interest statement
Competing Interest Statement: No competing interests
Figures

Similar articles
-
AgRP neurons mediate activity-dependent development of oxytocin connectivity and autonomic regulation.bioRxiv [Preprint]. 2024 Jun 3:2024.06.02.592838. doi: 10.1101/2024.06.02.592838. bioRxiv. 2024. PMID: 38895484 Free PMC article. Preprint.
-
Brain-derived neurotrophic factor is required for axonal growth of selective groups of neurons in the arcuate nucleus.Mol Metab. 2015 Mar 20;4(6):471-82. doi: 10.1016/j.molmet.2015.03.003. eCollection 2015 Jun. Mol Metab. 2015. PMID: 26042201 Free PMC article.
-
Microglial and peripheral immune priming is partially sexually dimorphic in adolescent mouse offspring exposed to maternal high-fat diet.J Neuroinflammation. 2020 Sep 5;17(1):264. doi: 10.1186/s12974-020-01914-1. J Neuroinflammation. 2020. PMID: 32891154 Free PMC article.
-
Neonatal leptin exposure specifies innervation of presympathetic hypothalamic neurons and improves the metabolic status of leptin-deficient mice.J Neurosci. 2013 Jan 9;33(2):840-51. doi: 10.1523/JNEUROSCI.3215-12.2013. J Neurosci. 2013. PMID: 23303959 Free PMC article.
-
Development of Hypothalamic Circuits That Control Food Intake and Energy Balance.In: Harris RBS, editor. Appetite and Food Intake: Central Control. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 7. In: Harris RBS, editor. Appetite and Food Intake: Central Control. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 7. PMID: 28880512 Free Books & Documents. Review.
References
-
- Akhaphong B., Gregg B., Kumusoglu D., Jo S., Singer K., Scheys J., DelProposto J., Lumeng C., Bernal-Mizrachi E., & Alejandro E. U. (2022). Maternal High-Fat Diet During Pre-Conception and Gestation Predisposes Adult Female Offspring to Metabolic Dysfunction in Mice. Frontiers in Endocrinology, 12, 780300. 10.3389/fendo.2021.780300 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
