Safety and potential effects of intrathecal injection of allogeneic human umbilical cord mesenchymal stem cell-derived exosomes in complete subacute spinal cord injury: a first-in-human, single-arm, open-label, phase I clinical trial
- PMID: 39183334
- PMCID: PMC11346059
- DOI: 10.1186/s13287-024-03868-0
Safety and potential effects of intrathecal injection of allogeneic human umbilical cord mesenchymal stem cell-derived exosomes in complete subacute spinal cord injury: a first-in-human, single-arm, open-label, phase I clinical trial
Abstract
Objective: Neurological and functional impairments are commonly observed in individuals with spinal cord injury (SCI) due to insufficient regeneration of damaged axons. Exosomes play a crucial role in the paracrine effects of mesenchymal stem cells (MSCs) and have emerged as a promising therapeutic approach for SCI. Thus, this study aimed to evaluate the safety and potential effects of intrathecal administration of allogeneic exosomes derived from human umbilical cord MSCs (HUC-MSCs) in patients with complete subacute SCI.
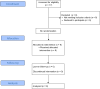
Methods: This study was a single-arm, open-label, phase I clinical trial with a 12-month follow-up period. HUC-MSCs were extracted from human umbilical cord tissue, and exosomes were isolated via ultracentrifugation. After intrathecal injection, each participant a underwent complete evaluation, including neurological assessment using the American Spinal Injury Association (ASIA) scale, functional assessment using the Spinal Cord Independence Measure (SCIM-III), neurogenic bowel dysfunction (NBD) assessment using the NBD score, modified Ashworth scale (MAS), and lower urinary tract function questionnaire.
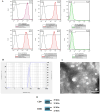
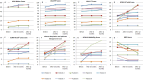
Results: Nine patients with complete subacute SCI were recruited. The intrathecal injection of allogeneic HUC-MSCs-exosomes was safe and well tolerated. No early or late adverse event (AE) attributable to the study intervention was observed. Significant improvements in ASIA pinprick (P-value = 0.039) and light touch (P-value = 0.038) scores, SCIM III total score (P-value = 0.027), and NBD score (P-value = 0.042) were also observed at 12-month after the injection compared with baseline.
Conclusions: This study demonstrated that intrathecal administration of allogeneic HUC-MSCs-exosomes is safe in patients with subacute SCI. Moreover, it seems that this therapy might be associated with potential clinical and functional improvements in these patients. In this regard, future larger phase II/III clinical trials with adequate power are highly required.
Trial registration: Iranian Registry of Clinical Trials, IRCT20200502047277N1. Registered 2 October 2020, https://en.irct.ir/trial/48765 .
Keywords: Exosomes; Mesenchymal stem cells; Neuroprotection; Spinal cord injury.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests .
Figures
Similar articles
-
Repeated subarachnoid administrations of allogeneic human umbilical cord mesenchymal stem cells for spinal cord injury: a phase 1/2 pilot study.Cytotherapy. 2021 Jan;23(1):57-64. doi: 10.1016/j.jcyt.2020.09.012. Epub 2020 Nov 18. Cytotherapy. 2021. PMID: 33218835
-
Safety and potential efficacy of expanded mesenchymal stromal cells of bone marrow and umbilical cord origins in patients with chronic spinal cord injuries: a phase I/II study.Cytotherapy. 2024 Aug;26(8):825-831. doi: 10.1016/j.jcyt.2024.03.480. Epub 2024 Apr 10. Cytotherapy. 2024. PMID: 38703153 Clinical Trial.
-
Combining cell therapy with human autologous Schwann cell and bone marrow-derived mesenchymal stem cell in patients with subacute complete spinal cord injury: safety considerations and possible outcomes.Stem Cell Res Ther. 2021 Aug 9;12(1):445. doi: 10.1186/s13287-021-02515-2. Stem Cell Res Ther. 2021. PMID: 34372939 Free PMC article.
-
Bone marrow stem cells and polymer hydrogels--two strategies for spinal cord injury repair.Cell Mol Neurobiol. 2006 Oct-Nov;26(7-8):1113-29. doi: 10.1007/s10571-006-9007-2. Epub 2006 Apr 22. Cell Mol Neurobiol. 2006. PMID: 16633897 Review.
-
Exosomes Derived from Mesenchymal Stem Cells: Therapeutic Opportunities for Spinal Cord Injury.Bull Exp Biol Med. 2024 Apr;176(6):716-721. doi: 10.1007/s10517-024-06095-y. Epub 2024 Jun 18. Bull Exp Biol Med. 2024. PMID: 38888648 Review.
References
-
- James SL, Theadom A, Ellenbogen RG, Bannick MS, Montjoy-Venning W, Lucchesi LR, et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet Neurol. 2019;18(1):56–87. 10.1016/S1474-4422(18)30415-0 - DOI - PMC - PubMed
-
- Ahuja CS, Wilson JR, Nori S, Kotter M, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Reviews Disease Primers. 2017;3(1):1–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

