Pathological characteristics of axons and alterations of proteomic and lipidomic profiles in midbrain dopaminergic neurodegeneration induced by WDR45-deficiency
- PMID: 39183331
- PMCID: PMC11346282
- DOI: 10.1186/s13024-024-00746-4
Pathological characteristics of axons and alterations of proteomic and lipidomic profiles in midbrain dopaminergic neurodegeneration induced by WDR45-deficiency
Abstract
Background: Although WD repeat domain 45 (WDR45) mutations have been linked to -propeller protein-associated neurodegeneration (BPAN), the precise molecular and cellular mechanisms behind this disease remain elusive. This study aims to shed light on the impacts of WDR45-deficiency on neurodegeneration, specifically axonal degeneration, within the midbrain dopaminergic (DAergic) system. We hope to better understand the disease process by examining pathological and molecular alterations, especially within the DAergic system.
Methods: To investigate the impacts of WDR45 dysfunction on mouse behaviors and DAergic neurons, we developed a mouse model in which WDR45 was conditionally knocked out in the midbrain DAergic neurons (WDR45cKO). Through a longitudinal study, we assessed alterations in the mouse behaviors using open field, rotarod, Y-maze, and 3-chamber social approach tests. We utilized a combination of immunofluorescence staining and transmission electron microscopy to examine the pathological changes in DAergic neuron soma and axons. Additionally, we performed proteomic and lipidomic analyses of the striatum from young and aged mice to identify the molecules and processes potentially involved in the striatal pathology during aging. Further more, primary midbrain neuronal culture was employed to explore the molecular mechanisms leading to axonal degeneration.
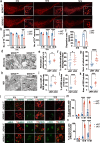
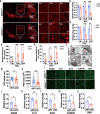
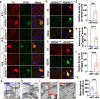
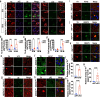
Results: Our study of WDR45cKO mice revealed a range of deficits, including impaired motor function, emotional instability, and memory loss, coinciding with the profound reduction of midbrain DAergic neurons. The neuronal loss, we observed massive axonal enlargements in the dorsal and ventral striatum. These enlargements were characterized by the accumulation of extensively fragmented tubular endoplasmic reticulum (ER), a hallmark of axonal degeneration. Proteomic analysis of the striatum showed that the differentially expressed proteins were enriched in metabolic processes. The carbohydrate metabolic and protein catabolic processes appeared earlier, and amino acid, lipid, and tricarboxylic acid metabolisms were increased during aging. Of note, we observed a tremendous increase in the expression of lysophosphatidylcholine acyltransferase 1 (Lpcat1) that regulates phospholipid metabolism, specifically in the conversion of lysophosphatidylcholine (LPC) to phosphatidylcholine (PC) in the presence of acyl-CoA. The lipidomic results consistently suggested that differential lipids were concentrated on PC and LPC. Axonal degeneration was effectively ameliorated by interfering Lpcat1 expression in primary cultured WDR45-deficient DAergic neurons, proving that Lpcat1 and its regulated lipid metabolism, especially PC and LPC metabolism, participate in controlling the axonal degeneration induced by WDR45 deficits.
Conclusions: In this study, we uncovered the molecular mechanisms underlying the contribution of WDR45 deficiency to axonal degeneration, which involves complex relationships between phospholipid metabolism, autophagy, and tubular ER. These findings greatly advance our understanding of the fundamental molecular mechanisms driving axonal degeneration and may provide a foundation for developing novel mechanistically based therapeutic interventions for BPAN and other neurodegenerative diseases.
Keywords: Autophagy; Axonal degeneration; Lpcat1; Phospholipid metabolism; Tubular ER; WDR45.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Update of
-
Pathological characteristics of axons and proteome patterns in midbrain dopaminergic neurodegeneration induced by WDR45-deficiency.Res Sq [Preprint]. 2023 May 18:rs.3.rs-2901370. doi: 10.21203/rs.3.rs-2901370/v1. Res Sq. 2023. Update in: Mol Neurodegener. 2024 Aug 26;19(1):62. doi: 10.1186/s13024-024-00746-4. PMID: 37292937 Free PMC article. Updated. Preprint.
Similar articles
-
Pathological characteristics of axons and proteome patterns in midbrain dopaminergic neurodegeneration induced by WDR45-deficiency.Res Sq [Preprint]. 2023 May 18:rs.3.rs-2901370. doi: 10.21203/rs.3.rs-2901370/v1. Res Sq. 2023. Update in: Mol Neurodegener. 2024 Aug 26;19(1):62. doi: 10.1186/s13024-024-00746-4. PMID: 37292937 Free PMC article. Updated. Preprint.
-
WDR45 contributes to neurodegeneration through regulation of ER homeostasis and neuronal death.Autophagy. 2020 Mar;16(3):531-547. doi: 10.1080/15548627.2019.1630224. Epub 2019 Jun 23. Autophagy. 2020. PMID: 31204559 Free PMC article.
-
The autophagy gene Wdr45/Wipi4 regulates learning and memory function and axonal homeostasis.Autophagy. 2015;11(6):881-90. doi: 10.1080/15548627.2015.1047127. Autophagy. 2015. PMID: 26000824 Free PMC article.
-
Evidence for dopaminergic axonal degeneration as an early pathological process in Parkinson's disease.Parkinsonism Relat Disord. 2018 Nov;56:9-15. doi: 10.1016/j.parkreldis.2018.06.025. Epub 2018 Jun 19. Parkinsonism Relat Disord. 2018. PMID: 29934196 Review.
-
A sporadic Parkinson's disease model via silencing of the ubiquitin-proteasome/E3 ligase component, SKP1A.J Neural Transm (Vienna). 2024 Jun;131(6):675-707. doi: 10.1007/s00702-023-02687-6. Epub 2023 Aug 29. J Neural Transm (Vienna). 2024. PMID: 37644186 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

