Schnurri-3 inhibition rescues skeletal fragility and vascular skeletal stem cell niche pathology in the OIM model of osteogenesis imperfecta
- PMID: 39183236
- PMCID: PMC11345453
- DOI: 10.1038/s41413-024-00349-1
Schnurri-3 inhibition rescues skeletal fragility and vascular skeletal stem cell niche pathology in the OIM model of osteogenesis imperfecta
Abstract
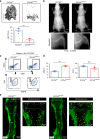
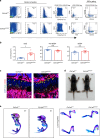
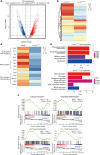
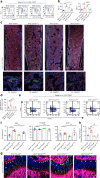
Osteogenesis imperfecta (OI) is a disorder of low bone mass and increased fracture risk due to a range of genetic variants that prominently include mutations in genes encoding type I collagen. While it is well known that OI reflects defects in the activity of bone-forming osteoblasts, it is currently unclear whether OI also reflects defects in the many other cell types comprising bone, including defects in skeletal vascular endothelium or the skeletal stem cell populations that give rise to osteoblasts and whether correcting these broader defects could have therapeutic utility. Here, we find that numbers of skeletal stem cells (SSCs) and skeletal arterial endothelial cells (AECs) are augmented in Col1a2oim/oim mice, a well-studied animal model of moderate to severe OI, suggesting that disruption of a vascular SSC niche is a feature of OI pathogenesis. Moreover, crossing Col1a2oim/oim mice to mice lacking a negative regulator of skeletal angiogenesis and bone formation, Schnurri 3 (SHN3), not only corrected the SSC and AEC phenotypes but moreover robustly corrected the bone mass and spontaneous fracture phenotypes. As this finding suggested a strong therapeutic utility of SHN3 inhibition for the treatment of OI, a bone-targeting AAV was used to mediate Shn3 knockdown, rescuing the Col1a2oim/oim phenotype and providing therapeutic proof-of-concept for targeting SHN3 for the treatment of OI. Overall, this work both provides proof-of-concept for inhibition of the SHN3 pathway and more broadly addressing defects in the stem/osteoprogenitor niche as is a strategy to treat OI.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Update of
-
Schnurri-3 inhibition rescues skeletal fragility and vascular skeletal stem cell niche pathology in a mouse model of osteogenesis imperfecta.Res Sq [Preprint]. 2023 Jul 26:rs.3.rs-3153957. doi: 10.21203/rs.3.rs-3153957/v1. Res Sq. 2023. Update in: Bone Res. 2024 Aug 26;12(1):46. doi: 10.1038/s41413-024-00349-1. PMID: 37546916 Free PMC article. Updated. Preprint.
Similar articles
-
Schnurri-3 inhibition rescues skeletal fragility and vascular skeletal stem cell niche pathology in a mouse model of osteogenesis imperfecta.Res Sq [Preprint]. 2023 Jul 26:rs.3.rs-3153957. doi: 10.21203/rs.3.rs-3153957/v1. Res Sq. 2023. Update in: Bone Res. 2024 Aug 26;12(1):46. doi: 10.1038/s41413-024-00349-1. PMID: 37546916 Free PMC article. Updated. Preprint.
-
Distinct type I collagen alterations cause intrinsic lung and respiratory defects of variable severity in mouse models of osteogenesis imperfecta.J Physiol. 2023 Jan;601(2):355-379. doi: 10.1113/JP283452. Epub 2022 Nov 9. J Physiol. 2023. PMID: 36285717 Free PMC article.
-
Galunisertib downregulates mutant type I collagen expression and promotes MSCs osteogenesis in pediatric osteogenesis imperfecta.Biomed Pharmacother. 2024 Jun;175:116725. doi: 10.1016/j.biopha.2024.116725. Epub 2024 May 13. Biomed Pharmacother. 2024. PMID: 38744219
-
OIM and related animal models of osteogenesis imperfecta.Connect Tissue Res. 1995;31(4):265-8. doi: 10.3109/03008209509010820. Connect Tissue Res. 1995. PMID: 15612365 Review.
-
Bone Quality and Mineralization and Effects of Treatment in Osteogenesis Imperfecta.Calcif Tissue Int. 2024 Sep 4. doi: 10.1007/s00223-024-01263-8. Online ahead of print. Calcif Tissue Int. 2024. PMID: 39231826 Review.
References
-
- Marini, J. C. et al. Osteogenesis imperfecta. Nat. Rev. Dis. Prim.3, 17052 (2017). - PubMed
-
- Rauch, F. & Glorieux, F. H. Osteogenesis imperfecta. Lancet363, 1377–1385 (2004). - PubMed
-
- Palomo, T. et al. Intravenous bisphosphonate therapy of young children with osteogenesis imperfecta: skeletal findings during follow up throughout the growing years. J. Bone Miner. Res.30, 2150–2157 (2015). - PubMed
-
- Glorieux, F. H. et al. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N. Engl. J. Med.339, 947–952 (1998). - PubMed
MeSH terms
Substances
Grants and funding
- 92068104/National Natural Science Foundation of China (National Science Foundation of China)
- 82002262/National Natural Science Foundation of China (National Science Foundation of China)
- R01 AR075585/AR/NIAMS NIH HHS/United States
- R01AR075585/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 HD115274/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

