Glucose-1,6-bisphosphate: A new gatekeeper of cerebral mitochondrial pyruvate uptake
- PMID: 39182844
- PMCID: PMC11404074
- DOI: 10.1016/j.molmet.2024.102018
Glucose-1,6-bisphosphate: A new gatekeeper of cerebral mitochondrial pyruvate uptake
Abstract
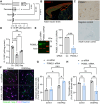
Objective: Glucose-1,6-bisphosphate (G-1,6-BP), a byproduct of glycolysis that is synthesized by phosphoglucomutase 2 like 1 (PGM2L1), is particularly abundant in neurons. G-1,6-BP is sensitive to the glycolytic flux, due to its dependence on 1,3-bisphosphoglycerate as phosphate donor, and the energy state, due to its degradation by inosine monophosphate-activated phosphomannomutase 1. Since the exact role of this metabolite remains unclear, our aim was to elucidate the specific function of G-1,6-BP in the brain.
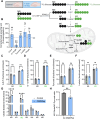
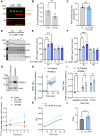
Methods: The effect of PGM2L1 on neuronal post-ischemic viability was assessed by siRNA-mediated knockdown of PGM2L1 in primary mouse neurons. Acute mouse brain slices were used to correlate the reduction in G-1,6-BP upon ischemia to changes in carbon metabolism by 13C6-glucose tracing. A drug affinity responsive target stability assay was used to test if G-1,6-BP interacts with the mitochondrial pyruvate carrier (MPC) subunits in mouse brain protein extracts. Human embryonic kidney cells expressing a MPC bioluminescence resonance energy transfer sensor were used to analyze how PGM2L1 overexpression affects MPC activity. The effect of G-1,6-BP on mitochondrial pyruvate uptake and oxygen consumption rates was analyzed in isolated mouse brain mitochondria. PGM2L1 and a predicted upstream kinase were overexpressed in a human neuroblastoma cell line and G-1,6-BP levels were measured.
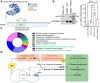
Results: We found that G-1,6-BP in mouse brain slices was quickly degraded upon ischemia and reperfusion. Knockdown of PGM2L1 in mouse neurons reduced post-ischemic viability, indicating that PGM2L1 plays a neuroprotective role. The reduction in G-1,6-BP upon ischemia was not accompanied by alterations in glycolytic rates but we did see a reduced 13C6-glucose incorporation into citrate, suggesting a potential role in mitochondrial pyruvate uptake or metabolism. Indeed, G-1,6-BP interacted with both MPC subunits and overexpression of PGM2L1 increased MPC activity. G-1,6-BP, at concentrations found in the brain, enhanced mitochondrial pyruvate uptake and pyruvate-induced oxygen consumption rates. Overexpression of a predicted upstream kinase inhibited PGM2L1 activity, showing that besides metabolism, also signaling pathways can regulate G-1,6-BP levels.
Conclusions: We provide evidence that G-1,6-BP positively regulates mitochondrial pyruvate uptake and post-ischemic neuronal viability. These compelling data reveal a novel mechanism by which neurons can couple glycolysis-derived pyruvate to the tricarboxylic acid cycle. This process is sensitive to the glycolytic flux, the cell's energetic state, and upstream signaling cascades, offering many regulatory means to fine-tune this critical metabolic step.
Keywords: Energy metabolism; Glucose-1,6-bisphosphate; Ischemia; Mitochondrial pyruvate carrier; Phosphoglucomutase 2 like 1; Protein kinase N1.
Copyright © 2024 The Authors. Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Protein kinase N1 deficiency results in upregulation of cerebral energy metabolism and is highly protective in in vivo and in vitro stroke models.Metabolism. 2024 Dec;161:156039. doi: 10.1016/j.metabol.2024.156039. Epub 2024 Sep 26. Metabolism. 2024. PMID: 39332493
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Misprogramming of glucose metabolism impairs recovery of hippocampal slices from neuronal GLT-1 knockout mice and contributes to excitotoxic injury through mitochondrial superoxide production.J Neurochem. 2025 Jan;169(1):e16205. doi: 10.1111/jnc.16205. Epub 2024 Aug 28. J Neurochem. 2025. PMID: 39193789
-
The effectiveness of abstinence-based and harm reduction-based interventions in reducing problematic substance use in adults who are experiencing homelessness in high income countries: A systematic review and meta-analysis: A systematic review.Campbell Syst Rev. 2024 Apr 21;20(2):e1396. doi: 10.1002/cl2.1396. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38645303 Free PMC article. Review.
References
-
- Dienel G.A. Brain glucose metabolism: integration of energetics with function. Physiol Rev. 2019;99(1):949–1045. - PubMed
-
- Rose I.A., Warms J.V., Kaklij G. A specific enzyme for glucose 1,6-bisphosphate synthesis. J Biol Chem. 1975;250(9):3466–3470. - PubMed
-
- Passonneau J.V., Lowry O.H., Schulz D.W., Brown J.G. Glucose 1,6-diphosphate formation by phosphoglucomutase in mammalian tissues. J Biol Chem. 1969;244(3):902–909. - PubMed
-
- Maliekal P., Sokolova T., Vertommen D., Veiga-da-Cunha M., Van Schaftingen E. Molecular identification of mammalian phosphopentomutase and glucose-1,6-bisphosphate synthase, two members of the α-D-phosphohexomutase family. J Biol Chem. 2007;282(44):31844–31851. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

