Age-associated temporal decline in butyrate-producing bacteria plays a key pathogenic role in the onset and progression of neuropathology and memory deficits in 3×Tg-AD mice
- PMID: 39182227
- PMCID: PMC11346541
- DOI: 10.1080/19490976.2024.2389319
Age-associated temporal decline in butyrate-producing bacteria plays a key pathogenic role in the onset and progression of neuropathology and memory deficits in 3×Tg-AD mice
Abstract
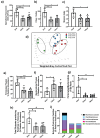
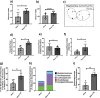
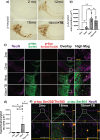
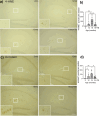
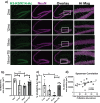
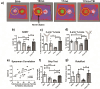
Alterations in the gut-microbiome-brain axis are increasingly being recognized to be involved in Alzheimer's disease (AD) pathogenesis. However, the functional consequences of enteric dysbiosis linking gut microbiota and brain pathology in AD progression remain largely undetermined. The present work investigated the causal role of age-associated temporal decline in butyrate-producing bacteria and butyrate in the etiopathogenesis of AD. Longitudinal metagenomics, neuropathological, and memory analyses were performed in the 3×Tg-AD mouse model. Metataxonomic analyses showed a significant temporal decline in the alpha diversity marked by a decrease in butyrate-producing bacterial communities and a concurrent reduction in cecal butyrate production. Inferred metagenomics analysis identified the bacterial acetyl-CoA pathway as the main butyrate synthesis pathway impacted. Concomitantly, there was an age-associated decline in the transcriptionally permissive acetylation of histone 3 at lysines 9 and 14 (H3K9/K14-Ac) in hippocampal neurons. Importantly, these microbiome-gut-brain changes preceded AD-related neuropathology, including oxidative stress, tau hyperphosphorylation, memory deficits, and neuromuscular dysfunction, which manifest by 17-18 months. Initiation of oral administration of tributyrin, a butyrate prodrug, at 6 months of age mitigated the age-related decline in butyrate-producing bacteria, protected the H3K9/K14-Ac status, and attenuated the development of neuropathological and cognitive changes associated with AD pathogenesis. These data causally implicate age-associated decline in butyrate-producing bacteria as a key pathogenic feature of the microbiome-gut-brain axis affecting the onset and progression of AD. Importantly, the regulation of butyrate-producing bacteria and consequent butyrate synthesis could be a significant therapeutic strategy in the prevention and treatment of AD.
Keywords: Alzheimer’s disease; butyrate-producing bacteria; gut dysbiosis; gut microbiome; histone acetylation; metagenomics; tau hyperphosphorylation; tributyrin.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Similar articles
-
Decrease in acetyl-CoA pathway utilizing butyrate-producing bacteria is a key pathogenic feature of alcohol-induced functional gut microbial dysbiosis and development of liver disease in mice.Gut Microbes. 2021 Jan-Dec;13(1):1946367. doi: 10.1080/19490976.2021.1946367. Gut Microbes. 2021. PMID: 34369304 Free PMC article.
-
Dietary Fiber Modulates the Release of Gut Bacterial Products Preventing Cognitive Decline in an Alzheimer's Mouse Model.Cell Mol Neurobiol. 2023 May;43(4):1595-1618. doi: 10.1007/s10571-022-01268-7. Epub 2022 Aug 11. Cell Mol Neurobiol. 2023. PMID: 35953741
-
Cultivable butyrate-producing bacteria of elderly Japanese diagnosed with Alzheimer's disease.J Microbiol. 2018 Oct;56(10):760-771. doi: 10.1007/s12275-018-8297-7. Epub 2018 Aug 22. J Microbiol. 2018. PMID: 30136260
-
Novelties on Neuroinflammation in Alzheimer's Disease-Focus on Gut and Oral Microbiota Involvement.Int J Mol Sci. 2024 Oct 19;25(20):11272. doi: 10.3390/ijms252011272. Int J Mol Sci. 2024. PMID: 39457054 Free PMC article. Review.
-
Gut Microbiome in Alzheimer's Disease: from Mice to Humans.Curr Neuropharmacol. 2024;22(14):2314-2329. doi: 10.2174/1570159X22666240308090741. Curr Neuropharmacol. 2024. PMID: 39403057 Free PMC article. Review.
References
-
- Grabrucker S, Marizzoni M, Silajdzic E, Lopizzo N, Mombelli E, Nicolas S, Dohm-Hansen S, Scassellati C, Moretti DV, Rosa M, et al. Microbiota from Alzheimer’s patients induce deficits in cognition and hippocampal neurogenesis. Brain. 2023;146(12):4916–4934. doi:10.1093/brain/awad303. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
