CAR Macrophages: a promising novel immunotherapy for solid tumors and beyond
- PMID: 39175095
- PMCID: PMC11342599
- DOI: 10.1186/s40364-024-00637-2
CAR Macrophages: a promising novel immunotherapy for solid tumors and beyond
Abstract
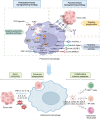
With the advent of adoptive cellular therapy, chimeric antigen receptor (CAR)-T cell therapy has gained widespread application in cancer treatment and has demonstrated significant efficacy against certain hematologic malignancies. However, due to the limitations of CAR-T cell therapy in treating solid tumors, other immune cells are being modified with CAR to address this issue. Macrophages have emerged as a promising option, owing to their extensive immune functions, which include antigen presentation, powerful tumor phagocytosis, and particularly active trafficking to the tumor microenvironment. Leveraging their unique advantages, CAR-macrophages (CAR-M) are expected to enhance the effectiveness of solid tumor treatments as a novel form of immunotherapy, potentially overcoming major challenges associated with CAR-T/NK therapy. This review outlines the primary mechanism underlying CAR-M and recent progressions in CAR-M therapy, while also discussing their further applications.
Keywords: CAR-Macrophages; Chimeric antigen receptor; Macrophages; Solid tumor.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Engineered CAR-Macrophages as Adoptive Immunotherapies for Solid Tumors.Front Immunol. 2021 Nov 24;12:783305. doi: 10.3389/fimmu.2021.783305. eCollection 2021. Front Immunol. 2021. PMID: 34899748 Free PMC article. Review.
-
CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy.J Exp Clin Cancer Res. 2022 Mar 31;41(1):119. doi: 10.1186/s13046-022-02327-z. J Exp Clin Cancer Res. 2022. PMID: 35361234 Free PMC article. Review.
-
CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances.Mol Cancer. 2023 Jan 30;22(1):20. doi: 10.1186/s12943-023-01723-z. Mol Cancer. 2023. PMID: 36717905 Free PMC article. Review.
-
Recent Advances in CAR-Based Solid Tumor Immunotherapy.Cells. 2023 Jun 11;12(12):1606. doi: 10.3390/cells12121606. Cells. 2023. PMID: 37371075 Free PMC article. Review.
-
CAR-macrophage: A new immunotherapy candidate against solid tumors.Biomed Pharmacother. 2021 Jul;139:111605. doi: 10.1016/j.biopha.2021.111605. Epub 2021 Apr 23. Biomed Pharmacother. 2021. PMID: 33901872 Review.
Cited by
-
Empowering brain tumor management: chimeric antigen receptor macrophage therapy.Theranostics. 2024 Sep 3;14(14):5725-5742. doi: 10.7150/thno.98290. eCollection 2024. Theranostics. 2024. PMID: 39310093 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

