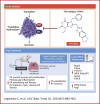
Novel Tryptophan Hydroxylase Inhibitor TPT-001 Reverses PAH, Vascular Remodeling, and Proliferative-Proinflammatory Gene Expression
- PMID: 39170954
- PMCID: PMC11334415
- DOI: 10.1016/j.jacbts.2024.04.006
Novel Tryptophan Hydroxylase Inhibitor TPT-001 Reverses PAH, Vascular Remodeling, and Proliferative-Proinflammatory Gene Expression
Abstract
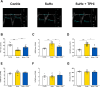
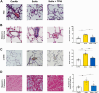
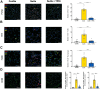
The serotonin pathway has long been proposed as a promising target for pulmonary arterial hypertension (PAH)-a progressive and uncurable disease. We developed a highly specific inhibitor of the serotonin synthesizing enzyme tryptophan hydroxylase 1 (TPH1), TPT-001 (TPHi). In this study, the authors sought to treat severe PAH in the Sugen/hypoxia (SuHx) rat model with the oral TPHi TPT-001. Male Sprague Dawley rats were divided into 3 groups: 1) ConNx, control animals; 2) SuHx, injected subcutaneously with SU5416 and exposed to chronic hypoxia for 3 weeks, followed by 6 weeks in room air; and 3) SuHx+TPHi, SuHx animals treated orally with TPHi for 5 weeks. Closed-chest right- and left heart catheterization and echocardiography were performed. Lungs were subject to histologic and mRNA sequencing analyses. Compared with SuHx-exposed rats, which developed severe PAH and right ventricular (RV) dysfunction, TPHi-treated SuHx rats had greatly lowered RV systolic (mean ± SEM: 41 ± 2.3 mm Hg vs 86 ± 6.5 mm Hg; P < 0.001) and end-diastolic (mean ± SEM: 4 ± 0.7 mm Hg vs 14 ± 1.7 mm Hg; P < 0.001) pressures, decreased RV hypertrophy and dilation (all not significantly different from control rats), and reversed pulmonary vascular remodeling. We identified perivascular infiltration of CD3+ T cells and proinflammatory F4/80+ and CD68+ macrophages and proliferating cell nuclear antigen-positive alveolar epithelial cells all suppressed by TPHi treatment. Whole-lung mRNA sequencing in SuHx rats showed distinct gene expression patterns related to pulmonary arterial smooth muscle cell proliferation (Rpph1, Lgals3, Gata4), reactive oxygen species, inflammation (Tnfsrf17, iNOS), and vasodilation (Pde1b, Kng1), which reversed expression with TPHi treatment. Inhibition of TPH1 with a new class of drugs (here, TPT-001) has the potential to attenuate or even reverse severe PAH and associated RV dysfunction in vivo by blocking the serotonin pathway.
Keywords: drug discovery; pulmonary arterial hypertension; serotonin; tryptophan hydroxylase inhibitor.
© 2024 The Authors.
Conflict of interest statement
This study was funded by the Federal Ministry of Education and Research (01KC2001B and 03VP08053 to Dr Hansmann; 01KC2001A, 03VP08051, and 16GW0298 to Dr Bader). Dr Hansmann also receives funding from the German Research Foundation (DFG KFO311 grant HA4348/6-2) and the European Pediatric Pulmonary Vascular Disease Network. Dr Nazaré has received funding from the Federal Ministry of Economic Affairs (ZIM grant 16KN073251). Drs Specker, Nazaré, Matthes, and Bader hold patents on the novel class of TPHi. Drs Specker, Wesolowski, and Bader are founders of Trypto Therapeutics GmbH. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


Similar articles
-
Sulforaphane prevents right ventricular injury and reduces pulmonary vascular remodeling in pulmonary arterial hypertension.Am J Physiol Heart Circ Physiol. 2020 Apr 1;318(4):H853-H866. doi: 10.1152/ajpheart.00321.2019. Epub 2020 Feb 28. Am J Physiol Heart Circ Physiol. 2020. PMID: 32108526
-
Increased MAO-A Activity Promotes Progression of Pulmonary Arterial Hypertension.Am J Respir Cell Mol Biol. 2021 Mar;64(3):331-343. doi: 10.1165/rcmb.2020-0105OC. Am J Respir Cell Mol Biol. 2021. PMID: 33264068
-
Pulmonary artery banding is a relevant model to study the right ventricular remodeling and dysfunction that occurs in pulmonary arterial hypertension.J Appl Physiol (1985). 2020 Aug 1;129(2):238-246. doi: 10.1152/japplphysiol.00148.2020. Epub 2020 Jul 9. J Appl Physiol (1985). 2020. PMID: 32644912
-
Sulforaphane Does Not Protect Right Ventricular Systolic and Diastolic Functions in Nrf2 Knockout Pulmonary Artery Hypertension Mice.Cardiovasc Drugs Ther. 2022 Jun;36(3):425-436. doi: 10.1007/s10557-022-07323-1. Epub 2022 Feb 14. Cardiovasc Drugs Ther. 2022. PMID: 35157168 Free PMC article.
-
Serotonin transporter is not required for the development of severe pulmonary hypertension in the Sugen hypoxia rat model.Am J Physiol Lung Cell Mol Physiol. 2015 Nov 15;309(10):L1164-73. doi: 10.1152/ajplung.00127.2015. Epub 2015 Sep 18. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26386116
References
LinkOut - more resources
Full Text Sources
Miscellaneous

