Effects of chronic treatment with metformin on brain glucose hypometabolism and central insulin actions in transgenic mice with tauopathy
- PMID: 39170185
- PMCID: PMC11337050
- DOI: 10.1016/j.heliyon.2024.e35752
Effects of chronic treatment with metformin on brain glucose hypometabolism and central insulin actions in transgenic mice with tauopathy
Abstract
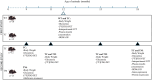
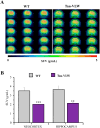
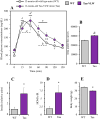
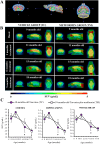
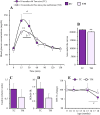
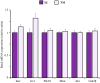
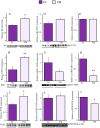
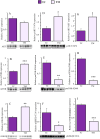
Brain glucose hypometabolism and insulin alterations are common features of many neurological diseases. Herein we sought to corroborate the brain glucose hypometabolism that develops with ageing in 12-months old Tau-VLW transgenic mice, a model of tauopathy, as well as to determine whether this model showed signs of altered peripheral glucose metabolism. Our results demonstrated that 12-old months Tau mice exhibited brain glucose hypometabolism as well as basal hyperglycemia, impaired glucose tolerance, hyperinsulinemia, and signs of insulin resistance. Then, we further studied the effect of chronic metformin treatment (9 months) in Tau-VLW mice from 9 to 18 months of age. Longitudinal PET neuroimaging studies revealed that chronic metformin altered the temporal profile in the progression of brain glucose hypometabolism associated with ageing. Besides, metformin altered the content and/or phosphorylation of key components of the insulin signal transduction pathway in the frontal cortex leading to significant changes in the content of the active forms. Thus, metformin increased the expression of pAKT-Y474 while reducing pmTOR-S2448 and pGSK3β. These changes might be related, at least partially, to a slow progression of ageing, neurological damage, and cognitive decline. Metformin also improved the peripheral glucose tolerance and the ability of the Tau-VLW mice to maintain their body weight through ageing. Altogether our study shows that the tau-VLW mice could be a useful model to study the potential interrelationship between tauopathy and central and peripheral glucose metabolism alterations. More importantly our results suggest that chronic metformin treatment may have direct beneficial central effects by post-transcriptional modulation of key components of the insulin signal transduction pathway.
Keywords: Brain; Glucose hypometabolism; Insulin resistance; Tauopathy; Transgenic mice.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Assessment of the In Vivo Relationship Between Cerebral Hypometabolism, Tau Deposition, TSPO Expression, and Synaptic Density in a Tauopathy Mouse Model: a Multi-tracer PET Study.Mol Neurobiol. 2022 Jun;59(6):3402-3413. doi: 10.1007/s12035-022-02793-8. Epub 2022 Mar 21. Mol Neurobiol. 2022. PMID: 35312967 Free PMC article.
-
The GABAergic septohippocampal connection is impaired in a mouse model of tauopathy.Neurobiol Aging. 2017 Jan;49:40-51. doi: 10.1016/j.neurobiolaging.2016.09.006. Epub 2016 Sep 15. Neurobiol Aging. 2017. PMID: 27743524
-
Metformin promotes tau aggregation and exacerbates abnormal behavior in a mouse model of tauopathy.Mol Neurodegener. 2016 Feb 9;11:16. doi: 10.1186/s13024-016-0082-7. Mol Neurodegener. 2016. PMID: 26858121 Free PMC article.
-
Significance of Brain Glucose Hypometabolism, Altered Insulin Signal Transduction, and Insulin Resistance in Several Neurological Diseases.Front Endocrinol (Lausanne). 2022 May 9;13:873301. doi: 10.3389/fendo.2022.873301. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35615716 Free PMC article. Review.
-
Decoding Alzheimer's disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies.Prog Neurobiol. 2013 Sep;108:21-43. doi: 10.1016/j.pneurobio.2013.06.004. Epub 2013 Jul 11. Prog Neurobiol. 2013. PMID: 23850509 Review.
References
-
- Blázquez E., Hurtado-Carneiro V., LeBaut-Ayuso Y., Velázquez E., García-García L., Gómez-Oliver F., Ruiz-Albusac J.M., Ávila J., Pozo M.Á. Significance of brain glucose hypometabolism, altered insulin signal transduction, and insulin resistance in several neurological diseases. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.873301. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

