Gut dysbiosis contributes to the development of Budd-Chiari syndrome through immune imbalance
- PMID: 39166878
- PMCID: PMC11406926
- DOI: 10.1128/msystems.00794-24
Gut dysbiosis contributes to the development of Budd-Chiari syndrome through immune imbalance
Abstract
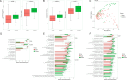
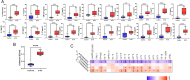
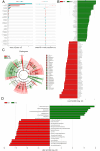
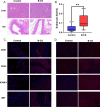
Budd-Chiari syndrome (B-CS) is a rare and lethal condition characterized by hepatic venous outflow tract blockage. Gut microbiota has been linked to numerous hepatic disorders, but its significance in B-CS pathogenesis is uncertain. First, we performed a case-control study (Ncase = 140, Ncontrol = 63) to compare the fecal microbiota of B-CS and healthy individuals by metagenomics sequencing. B-CS patients' gut microbial composition and activity changed significantly, with a different metagenomic makeup, increased potentially pathogenic bacteria, including Prevotella, and disease-linked microbial function. Imbalanced cytokines in patients were demonstrated to be associated with gut dysbiosis, which led us to suspect that B-CS is associated with gut microbiota and immune dysregulation. Next, 16S ribosomal DNA sequencing on fecal microbiota transplantation (FMT) mice models examined the link between gut dysbiosis and B-CS. FMT models showed damaged liver tissues, posterior inferior vena cava, and increased Prevotella in the disturbed gut microbiota of FMT mice. Notably, B-CS-FMT impaired the morphological structure of colonic tissues and increased intestinal permeability. Furthermore, a significant increase of the same cytokines (IL-5, IL-6, IL-9, IL-10, IL-17A, IL-17F, and IL-13) and endotoxin levels in B-CS-FMT mice were observed. Our study suggested that gut microbial dysbiosis may cause B-CS through immunological dysregulation.
Importance: This study revealed that gut microbial dysbiosis may cause Budd-Chiari syndrome (B-CS). Gut dysbiosis enhanced intestinal permeability, and toxic metabolites and imbalanced cytokines activated the immune system. Consequently, the escalation of causative factors led to their concentration in the portal vein, thereby compromising both the liver parenchyma and outflow tract. Therefore, we proposed that gut microbial dysbiosis induced immune imbalance by chronic systemic inflammation, which contributed to the B-CS development. Furthermore, Prevotella may mediate inflammation development and immune imbalance, showing potential in B-CS pathogenesis.
Keywords: Budd-Chiari syndrome; fecal microbiota transplantation; gut microbiota; gut microbiota dysbiosis; immune imbalance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Early-Life Intervention Using Fecal Microbiota Combined with Probiotics Promotes Gut Microbiota Maturation, Regulates Immune System Development, and Alleviates Weaning Stress in Piglets.Int J Mol Sci. 2020 Jan 13;21(2):503. doi: 10.3390/ijms21020503. Int J Mol Sci. 2020. PMID: 31941102 Free PMC article.
-
Gut microbiota dysbiosis worsens the severity of acute pancreatitis in patients and mice.J Gastroenterol. 2019 Apr;54(4):347-358. doi: 10.1007/s00535-018-1529-0. Epub 2018 Dec 5. J Gastroenterol. 2019. PMID: 30519748
-
Unraveling the role of gut microbiota by fecal microbiota transplantation in rat model of kidney stone disease.Sci Rep. 2024 Sep 20;14(1):21924. doi: 10.1038/s41598-024-72694-4. Sci Rep. 2024. PMID: 39300177 Free PMC article.
-
Gut microbiota alteration and modulation in psychiatric disorders: Current evidence on fecal microbiota transplantation.Prog Neuropsychopharmacol Biol Psychiatry. 2021 Jul 13;109:110258. doi: 10.1016/j.pnpbp.2021.110258. Epub 2021 Jan 23. Prog Neuropsychopharmacol Biol Psychiatry. 2021. PMID: 33497754 Review.
-
Benefits of fecal microbiota transplantation: A comprehensive review.J Infect Dev Ctries. 2020 Oct 31;14(10):1074-1080. doi: 10.3855/jidc.12780. J Infect Dev Ctries. 2020. PMID: 33175698 Review.
References
-
- Northup PG, Garcia-Pagan JC, Garcia-Tsao G, Intagliata NM, Superina RA, Roberts LN, Lisman T, Valla DC. 2021. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 practice guidance by the American association for the study of liver diseases. Hepatology 73:366–413. doi:10.1002/hep.31646 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

