Neuroprotective and anti-inflammatory properties of proteins secreted by glial progenitor cells derived from human iPSCs
- PMID: 39165834
- PMCID: PMC11333358
- DOI: 10.3389/fncel.2024.1449063
Neuroprotective and anti-inflammatory properties of proteins secreted by glial progenitor cells derived from human iPSCs
Abstract
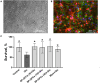
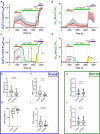
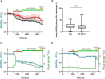
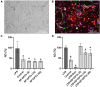
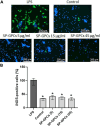
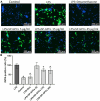
Currently, stem cells technology is an effective tool in regenerative medicine. Cell therapy is based on the use of stem/progenitor cells to repair or replace damaged tissues or organs. This approach can be used to treat various diseases, such as cardiovascular, neurological diseases, and injuries of various origins. The mechanisms of cell therapy therapeutic action are based on the integration of the graft into the damaged tissue (replacement effect) and the ability of cells to secrete biologically active molecules such as cytokines, growth factors and other signaling molecules that promote regeneration (paracrine effect). However, cell transplantation has a number of limitations due to cell transportation complexity and immune rejection. A potentially more effective therapy is using only paracrine factors released by stem cells. Secreted factors can positively affect the damaged tissue: promote forming new blood vessels, stimulate cell proliferation, and reduce inflammation and apoptosis. In this work, we have studied the anti-inflammatory and neuroprotective effects of proteins with a molecular weight below 100 kDa secreted by glial progenitor cells obtained from human induced pluripotent stem cells. Proteins secreted by glial progenitor cells exerted anti-inflammatory effects in a primary glial culture model of LPS-induced inflammation by reducing nitric oxide (NO) production through inhibition of inducible NO synthase (iNOS). At the same time, added secreted proteins neutralized the effect of glutamate, increasing the number of viable neurons to control values. This effect is a result of decreased level of intracellular calcium, which, at elevated concentrations, triggers apoptotic death of neurons. In addition, secreted proteins reduce mitochondrial depolarization caused by glutamate excitotoxicity and help maintain higher NADH levels. This therapy can be successfully introduced into clinical practice after additional preclinical studies, increasing the effectiveness of rehabilitation of patients with neurological diseases.
Keywords: LPS-induced inflammation; glial progenitor cells; glutamate excitotoxicity; human induced pluripotent stem cells; secreted proteins.
Copyright © 2024 Salikhova, Shedenkova, Sudina, Belousova, Krasilnikova, Nekrasova, Nefedova, Frolov, Fatkhudinov, Makarov, Surin, Savostyanov, Goldshtein and Bakaeva.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Therapeutic Effects of hiPSC-Derived Glial and Neuronal Progenitor Cells-Conditioned Medium in Experimental Ischemic Stroke in Rats.Int J Mol Sci. 2021 Apr 29;22(9):4694. doi: 10.3390/ijms22094694. Int J Mol Sci. 2021. PMID: 33946667 Free PMC article.
-
Neurotrophic Factors Secreted by Induced Pluripotent Stem Cell-Derived Retinal Progenitors Promote Retinal Survival and Preservation in an Adult Porcine Neuroretina Model.J Ocul Pharmacol Ther. 2021 Jun;37(5):301-312. doi: 10.1089/jop.2020.0088. Epub 2021 Mar 3. J Ocul Pharmacol Ther. 2021. PMID: 33661042
-
Therapeutic Efficiency of Proteins Secreted by Glial Progenitor Cells in a Rat Model of Traumatic Brain Injury.Int J Mol Sci. 2023 Aug 2;24(15):12341. doi: 10.3390/ijms241512341. Int J Mol Sci. 2023. PMID: 37569717 Free PMC article.
-
Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies?Leukemia. 2012 Jun;26(6):1166-73. doi: 10.1038/leu.2011.389. Epub 2011 Dec 19. Leukemia. 2012. PMID: 22182853 Review.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
References
-
- Bakaeva Z., Lizunova N., Tarzhanov I., Boyarkin D., Petrichuk S., Pinelis V., et al. (2022). Lipopolysaccharide from E. coli increases glutamate-induced disturbances of calcium homeostasis, the functional state of mitochondria, and the death of cultured cortical neurons. Front. Mol. Neurosci. 14:811171. 10.3389/fnmol.2021.811171 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

