An atlas of Brachypodium distachyon lateral root development
- PMID: 39158386
- PMCID: PMC11391822
- DOI: 10.1242/bio.060531
An atlas of Brachypodium distachyon lateral root development
Abstract
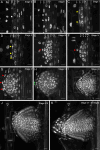
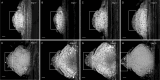
The root system of plants is a vital part for successful development and adaptation to different soil types and environments. A major determinant of the shape of a plant root system is the formation of lateral roots, allowing for expansion of the root system. Arabidopsis thaliana, with its simple root anatomy, has been extensively studied to reveal the genetic program underlying root branching. However, to get a more general understanding of lateral root development, comparative studies in species with a more complex root anatomy are required. Here, by combining optimized clearing methods and histology, we describe an atlas of lateral root development in Brachypodium distachyon, a wild, temperate grass species. We show that lateral roots initiate from enlarged phloem pole pericycle cells and that the overlying endodermis reactivates its cell cycle and eventually forms the root cap. In addition, auxin signaling reported by the DR5 reporter was not detected in the phloem pole pericycle cells or young primordia. In contrast, auxin signaling was activated in the overlying cortical cell layers, including the exodermis. Thus, Brachypodium is a valuable model to investigate how signaling pathways and cellular responses have been repurposed to facilitate lateral root organogenesis.
Keywords: Brachypodium distachyon; Endodermis; Exodermis; Lateral roots; Organogenesis.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



Similar articles
-
Disturbed local auxin homeostasis enhances cellular anisotropy and reveals alternative wiring of auxin-ethylene crosstalk in Brachypodium distachyon seminal roots.PLoS Genet. 2013 Jun;9(6):e1003564. doi: 10.1371/journal.pgen.1003564. Epub 2013 Jun 20. PLoS Genet. 2013. PMID: 23840182 Free PMC article.
-
Lipo-chitooligosaccharides promote lateral root formation and modify auxin homeostasis in Brachypodium distachyon.New Phytol. 2019 Mar;221(4):2190-2202. doi: 10.1111/nph.15551. Epub 2018 Nov 24. New Phytol. 2019. PMID: 30347445
-
Auxin mediates the touch-induced mechanical stimulation of adventitious root formation under windy conditions in Brachypodium distachyon.BMC Plant Biol. 2020 Jul 16;20(1):335. doi: 10.1186/s12870-020-02544-8. BMC Plant Biol. 2020. PMID: 32678030 Free PMC article.
-
Post-embryonic root organogenesis in cereals: branching out from model plants.Trends Plant Sci. 2013 Aug;18(8):459-67. doi: 10.1016/j.tplants.2013.04.010. Epub 2013 May 31. Trends Plant Sci. 2013. PMID: 23727199 Review.
-
Application of Brachypodium to the genetic improvement of wheat roots.J Exp Bot. 2012 May;63(9):3467-74. doi: 10.1093/jxb/ers044. Epub 2012 Mar 30. J Exp Bot. 2012. PMID: 22467408 Review.
References
-
- Bao, Y., Aggarwal, P., Robbins, N. E., Sturrock, C. J., Thompson, M. C., Tan, H. Q., Tham, C., Duan, L., Rodriguez, P. L., Vernoux, T.et al. (2014). Plant roots use a patterning mechanism to position lateral root branches toward available water. Proc. Natl Acad. Sci. USA 111, 9319-9324. 10.1073/pnas.1400966111 - DOI - PMC - PubMed
-
- Barberon, M., Vermeer, J. E. M., De Bellis, D., Wang, P., Naseer, S., Andersen, T. G., Humbel, B. M., Nawrath, C., Takano, J., Salt, D.et al. (2016). Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164, 447-459. 10.1016/j.cell.2015.12.021 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

