Health Risk Assessment of Metals in Antidiabetic Herbal Preparations: A Safety Screening
- PMID: 39145043
- PMCID: PMC11324365
- DOI: 10.1155/2024/6507185
Health Risk Assessment of Metals in Antidiabetic Herbal Preparations: A Safety Screening
Abstract
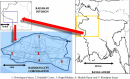
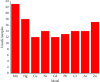
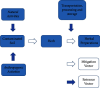
The present study evaluates the human health risk of metals in locally consumed herbal preparations used to treat diabetes. Atomic absorption spectroscopy (AAS) was used after microwave-assisted digestion to mineralize the samples. Toxic metal assessment was done by adopting mathematical modeling for carcinogenic and noncarcinogenic risks in the exposed population and comparing the raw results with maximum residue limits (MRLs) set by regulatory authorities. Hazard quotient (HQ) values for Fe, Hg, Cu, Pb, and Zn were recorded above 1. Noncarcinogenic health risks remain in 29% of samples for Fe, 67% of samples for Hg, 17% of samples for Cu, 33% of samples for Pb, and 4% of samples for Zn. Hazard index (HI) values in 33% of samples were above 1. Carcinogenic risks for Pb, Cr, Cd, and Ni were higher than the acceptable limit (1 × 10-6). Carcinogenic health risks exist in 54% of samples for Pb, 58% of samples for Cr, 46% of samples for Cd, and 58% of samples for Ni. MRLs for metals were crossed in samples in varying degrees. This is a harrowing account and may put public health safety at risk. Considering these facts, there should be more investigation into toxic metals in other frequently marketed herbal drugs in the antidiabetic and other therapeutic classes. Pre- and postmarket monitoring strategies for the preparations should also be in place to ensure safe consumption.
Copyright © 2024 Nazmul Islam et al.
Conflict of interest statement
The authors declare no conflicts of interest regarding the publication of the manuscript.
Figures
Similar articles
-
Honey Bee Products as Bio Indicator of Heavy Metals Pollution and Health Risk Assessment Through the Consumption of Multifloral Honey Collected in Azad Kashmir, Pakistan.Biol Trace Elem Res. 2025 Apr;203(4):2099-2113. doi: 10.1007/s12011-024-04313-2. Epub 2024 Jul 27. Biol Trace Elem Res. 2025. PMID: 39066963
-
Heavy metal levels and human health risk implications associated with fish consumption from the lower Omo river (Lotic) and Omo delta lake (Lentic), Ethiopia.PeerJ. 2024 Apr 29;12:e17216. doi: 10.7717/peerj.17216. eCollection 2024. PeerJ. 2024. PMID: 38699190 Free PMC article.
-
Analysis of heavy metal content in edible honeysuckle (Lonicera japonica Thunb.) from China and health risk assessment.J Environ Sci Health B. 2020;55(10):921-928. doi: 10.1080/03601234.2020.1797426. Epub 2020 Jul 28. J Environ Sci Health B. 2020. PMID: 32720560
-
Health Risk Assessment of Toxic Metal(loids) Consumed Through Plant-Based Anti-diabetic Therapeutics Collected in the Northern Divisional City of Rajshahi, Bangladesh.Biol Trace Elem Res. 2025 Apr;203(4):2149-2158. doi: 10.1007/s12011-024-04338-7. Epub 2024 Aug 12. Biol Trace Elem Res. 2025. PMID: 39129053
-
Human health risk assessment of edible body parts of chicken through heavy metals and trace elements quantitative analysis.PLoS One. 2023 Mar 10;18(3):e0279043. doi: 10.1371/journal.pone.0279043. eCollection 2023. PLoS One. 2023. PMID: 36897857 Free PMC article.
References
-
- Padmavathi P. Drug delivery system in nano greens. International Journal of Herbal Medicine . 2013;1(3):56–60.
-
- Bandaranayake W. M. Quality control, screening, toxicity, and regulation of herbal drugs in Modern Phytomedicine. In: Ahmad I., Aqil F., Owais M., editors. Turning Medicinal Plants into Drugs . Hoboken, NJ, USA: Whiley; 2006.
LinkOut - more resources
Full Text Sources