Group of longitudinal adverse event patterns after the fourth dose of COVID-19 vaccination with a latent class analysis
- PMID: 39139673
- PMCID: PMC11320210
- DOI: 10.3389/fpubh.2024.1406315
Group of longitudinal adverse event patterns after the fourth dose of COVID-19 vaccination with a latent class analysis
Abstract
Introduction: Vaccination has been implemented as a useful measure to combat the COVID-19 pandemic. However, there is a tendency for individuals to avoid vaccination due to the possibility of adverse events, making it important to investigate the relationship between COVID-19 vaccines and their adverse events. This study explored longitudinal adverse event patterns and factors that influence adverse events following the second to fourth doses of the COVID-19 vaccine through a latent class analysis.
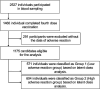
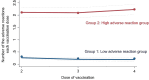
Methods: Participants were recruited from the Fukushima Prefecture and included individuals who had completed four doses of the COVID-19 mRNA vaccine. This study utilized data from questionnaire surveys and blood collection conducted between September 2021 and November 2022. In the questionnaire, factors such as sex, age, medical history, medication, type of vaccine administered, and adverse events following vaccination were recorded. Additionally, in the blood data, serological tests [IgG(S)] and cellular immune responses (T-spot) were measured. Descriptive statistics, latent class analysis, multivariable logistic regression, and multiple regression analyses were performed to identify the longitudinal adverse event patterns and influencing factors. By analyzing adverse events over time, we identified two distinct groups: those less prone to experiencing adverse events (Group 1) and those more susceptible (Group 2) to latent class analysis.
Results: A total of 1,175 participants were included after excluding those without any adverse events. The median age of the participants in Group 1 was 70 years, and in Group 2 it was 51 years. The proportion of female participants was 298 in Group 1 and 353 in Group 2. Patients in Group 2 were significantly younger (p < 0.001) and more likely to be female (p < 0.001) than those in Group 1. Furthermore, the median IgG(S) value after the fourth vaccination was 3,233 AU/mL in Group 1 and 4,059.39 AU/mL in Group 2. The median T-spot value was 15.4 in Group 1 and 28.5 in Group 2. Group 2 showed significantly higher IgG(S) and T-spot values after the fourth vaccination (p < 0.001).
Discussion: Our findings suggest that factors other than age, particularly sex and a history of allergies, significantly influence the likelihood of experiencing adverse events. Groups categorized by latent class analysis for longitudinal adverse events are expected to be valuable for optimizing vaccination strategies and formulating public health measures.
Keywords: COVID-19; Fukushima cohort; adverse events; latent class analysis; vaccination.
Copyright © 2024 Yamamoto, Kobashi, Kawamura, Nishikawa, Saito, Oguro, Zhao, Takita, Sawano, Ozaki, Abe, Ito, Kaneko, Nakayama, Wakui, Kodama and Tsubokura.
Conflict of interest statement
YKa was hired by Medical & Biological Laboratories Co. (MBL, Tokyo, Japan). MBL imported the test materials used in the study. YKa participated in the testing process; however, he did not engage in the research design and analysis. YKo and MTs received a grant from the Pfizer Health Research Foundation for research that was not associated with this study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Surveillance of Safety of 3 Doses of COVID-19 mRNA Vaccination Using Electronic Health Records.JAMA Netw Open. 2022 Apr 1;5(4):e227038. doi: 10.1001/jamanetworkopen.2022.7038. JAMA Netw Open. 2022. PMID: 35420661 Free PMC article.
-
Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial.Lancet. 2021 Dec 19;396(10267):1979-1993. doi: 10.1016/S0140-6736(20)32466-1. Epub 2020 Nov 19. Lancet. 2021. PMID: 33220855 Free PMC article. Clinical Trial.
-
BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers.Lancet Respir Med. 2021 Sep;9(9):999-1009. doi: 10.1016/S2213-2600(21)00220-4. Epub 2021 Jul 2. Lancet Respir Med. 2021. PMID: 34224675 Free PMC article.
-
Sex and gender differences in adverse events following influenza and COVID-19 vaccination.Biol Sex Differ. 2024 Jun 18;15(1):50. doi: 10.1186/s13293-024-00625-z. Biol Sex Differ. 2024. PMID: 38890702 Free PMC article.
References
-
- Roy DN, Azam MS, Islam E. Multi-dimensional potential factors influencing COVID-19 vaccine booster acceptance and hesitancy among university academic community in Bangladesh: a cross-sectional comparative study. PLoS One. (2023) 18:e0281395. doi: 10.1371/journal.pone.0281395, PMID: - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

