Improving the response to lenvatinib in partial responders using a Constrained-Disorder-Principle-based second-generation artificial intelligence-therapeutic regimen: a proof-of-concept open-labeled clinical trial
- PMID: 39139285
- PMCID: PMC11319816
- DOI: 10.3389/fonc.2024.1426426
Improving the response to lenvatinib in partial responders using a Constrained-Disorder-Principle-based second-generation artificial intelligence-therapeutic regimen: a proof-of-concept open-labeled clinical trial
Abstract
Introduction: The main obstacle in treating cancer patients is drug resistance. Lenvatinib treatment poses challenges due to loss of response and the common dose-limiting adverse events (AEs). The Constrained-disorder-principle (CDP)-based second-generation artificial intelligence (AI) systems introduce variability into treatment regimens and offer a potential strategy for enhancing treatment efficacy. This proof-of-concept clinical trial aimed to assess the impact of a personalized algorithm-controlled therapeutic regimen on lenvatinib effectiveness and tolerability.
Methods: A 14-week open-label, non-randomized trial was conducted with five cancer patients receiving lenvatinib-an AI-assisted application tailored to a personalized therapeutic regimen for each patient, which the treating physician approved. The study assessed changes in tumor response through FDG-PET-CT and tumor markers and quality of life via the EORTC QLQ-THY34 questionnaire, AEs, and laboratory evaluations. The app monitored treatment adherence.
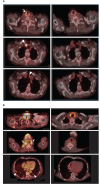
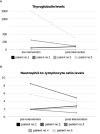
Results: At 14 weeks of follow-up, the disease control rate (including the following outcomes: complete response, partial response, stable disease) was 80%. The FDG-PET-CT scan-based RECIST v1.1 and PERCIST criteria showed partial response in 40% of patients and stable disease in an additional 40% of patients. One patient experienced a progressing disease. Of the participants with thyroid cancer, 75% showed a reduction in thyroglobulin levels, and 60% of all the participants showed a decrease in neutrophil-to-lymphocyte ratio during treatment. Improvement in the median social support score among patients utilizing the system supports an ancillary benefit of the intervention. No grade 4 AEs or functional deteriorations were recorded.
Summary: The results of this proof-of-concept open-labeled clinical trial suggest that the CDP-based second-generation AI system-generated personalized therapeutic recommendations may improve the response to lenvatinib with manageable AEs. Prospective controlled studies are needed to determine the efficacy of this approach.
Keywords: artificial intelligence; drug-resistant cancer; lenvatinib; salivary gland cancer; thyroid cancer.
Copyright © 2024 Sigawi, Gelman, Maimon, Yossef, Hemed, Agus, Berg, Ilan and Popovtzer.
Conflict of interest statement
YI is the founder of Oberon Sciences, SA is a consultant for Oberon Sciences, and MB is a consultant for Area9. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A second-generation artificial intelligence-based therapeutic regimen improves diuretic resistance in heart failure: Results of a feasibility open-labeled clinical trial.Biomed Pharmacother. 2023 May;161:114334. doi: 10.1016/j.biopha.2023.114334. Epub 2023 Mar 9. Biomed Pharmacother. 2023. PMID: 36905809 Clinical Trial.
-
A Feasibility Open-Labeled Clinical Trial Using a Second-Generation Artificial-Intelligence-Based Therapeutic Regimen in Patients with Gaucher Disease Treated with Enzyme Replacement Therapy.J Clin Med. 2024 Jun 5;13(11):3325. doi: 10.3390/jcm13113325. J Clin Med. 2024. PMID: 38893036 Free PMC article.
-
Assessment of Treatment Response to Lenvatinib in Thyroid Cancer Monitored by F-18 FDG PET/CT Using PERCIST 1.0, Modified PERCIST and EORTC Criteria-Which One Is Most Suitable?Cancers (Basel). 2022 Apr 7;14(8):1868. doi: 10.3390/cancers14081868. Cancers (Basel). 2022. PMID: 35454777 Free PMC article.
-
Optimisation of treatment with lenvatinib in radioactive iodine-refractory differentiated thyroid cancer.Cancer Treat Rev. 2018 Sep;69:164-176. doi: 10.1016/j.ctrv.2018.06.019. Epub 2018 Jul 2. Cancer Treat Rev. 2018. PMID: 30032061 Review.
-
Managing the adverse events associated with lenvatinib therapy in radioiodine-refractory differentiated thyroid cancer.Semin Oncol. 2019 Feb;46(1):57-64. doi: 10.1053/j.seminoncol.2018.11.004. Epub 2018 Dec 21. Semin Oncol. 2019. PMID: 30685073 Review.
Cited by
-
Harnessing Variability Signatures and Biological Noise May Enhance Immunotherapies' Efficacy and Act as Novel Biomarkers for Diagnosing and Monitoring Immune-Associated Disorders.Immunotargets Ther. 2024 Oct 14;13:525-539. doi: 10.2147/ITT.S477841. eCollection 2024. Immunotargets Ther. 2024. PMID: 39431244 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

