Activity-based protein profiling and global proteome analysis reveal MASTL as a potential therapeutic target in gastric cancer
- PMID: 39138495
- PMCID: PMC11323684
- DOI: 10.1186/s12964-024-01783-8
Activity-based protein profiling and global proteome analysis reveal MASTL as a potential therapeutic target in gastric cancer
Abstract
Background: Gastric cancer (GC) is a prevalent malignancy with limited therapeutic options for advanced stages. This study aimed to identify novel therapeutic targets for GC by profiling HSP90 client kinases.
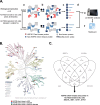
Methods: We used mass spectrometry-based activity-based protein profiling (ABPP) with a desthiobiotin-ATP probe, combined with sensitivity analysis of HSP90 inhibitors, to profile kinases in a panel of GC cell lines. We identified kinases regulated by HSP90 in inhibitor-sensitive cells and investigated the impact of MASTL knockdown on GC cell behavior. Global proteomic analysis following MASTL knockdown was performed, and bioinformatics tools were used to analyze the resulting data.
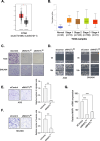
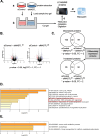
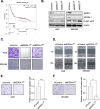
Results: Four kinases-MASTL, STK11, CHEK1, and MET-were identified as HSP90-regulated in HSP90 inhibitor-sensitive cells. Among these, microtubule-associated serine/threonine kinase-like (MASTL) was upregulated in GC and associated with poor prognosis. MASTL knockdown decreased migration, invasion, and proliferation of GC cells. Global proteomic profiling following MASTL knockdown revealed NEDD4-1 as a potential downstream mediator of MASTL in GC progression. NEDD4-1 was also upregulated in GC and associated with poor prognosis. Similar to MASTL inhibition, NEDD4-1 knockdown suppressed migration, invasion, and proliferation of GC cells.
Conclusions: Our multi-proteomic analyses suggest that targeting MASTL could be a promising therapy for advanced gastric cancer, potentially through the reduction of tumor-promoting proteins including NEDD4-1. This study enhances our understanding of kinase signaling pathways in GC and provides new insights for potential treatment strategies.
Keywords: Activity-based Protein Profiling; Gastric cancer; MASTL; NEDD4-1; Proteomics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Knockdown of Microtubule Associated Serine/threonine Kinase Like Expression Inhibits Gastric Cancer Cell Growth and Induces Apoptosis by Activation of ERK1/2 and Inactivation of NF-κB Signaling.Curr Med Sci. 2021 Feb;41(1):108-117. doi: 10.1007/s11596-021-2325-2. Epub 2021 Feb 13. Curr Med Sci. 2021. PMID: 33582914
-
Activity-Based Protein Profiling Reveals Potential Dasatinib Targets in Gastric Cancer.Int J Mol Sci. 2020 Dec 4;21(23):9276. doi: 10.3390/ijms21239276. Int J Mol Sci. 2020. PMID: 33291786 Free PMC article.
-
MASTL promotes cell contractility and motility through kinase-independent signaling.J Cell Biol. 2020 Jun 1;219(6):e201906204. doi: 10.1083/jcb.201906204. J Cell Biol. 2020. PMID: 32311005 Free PMC article.
-
MASTL: A novel therapeutic target for Cancer Malignancy.Cancer Med. 2020 Sep;9(17):6322-6329. doi: 10.1002/cam4.3141. Epub 2020 Jul 21. Cancer Med. 2020. PMID: 32692487 Free PMC article. Review.
-
Kinase-Independent Functions of MASTL in Cancer: A New Perspective on MASTL Targeting.Cells. 2020 Jul 6;9(7):1624. doi: 10.3390/cells9071624. Cells. 2020. PMID: 32640605 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

